Evaluation of Antiulcer Activity of Peltophorum Pterocarpum
Bhupalam Pradeepkumar1, CP Bhavyamadhuri2, Y Padmanabhareddy3, KV Veerabhadrappa4, Gorantla Narayana5, C Haranath6, K Somasekharreddy7, Akkiraju Sudheer8
1 Associate Professor and Head, Department of Pharmacy Practice, Raghavendra Institute of Pharmaceutical Education and Research (RIPER), Anantapur, Andhra Pradesh, India.
2 Pharmacy Student, Department of Pharmacy Practice, Raghavendra Institute of Pharmaceutical Education and Research (RIPER), Anantapur, Andhra Pradesh, India.
3 Principal, Department of Pharmaceutical Analysis, Raghavendra Institute of Pharmaceutical Education and Research (RIPER), Anantapur, Andhra Pradesh, India.
4 Associate Professor, Department of Pharmacognasy, Raghavendra Institute of Pharmaceutical Education and Research (RIPER), Anantapur, Andhra Pradesh, India.
5 Associate Professor, Department of Pharmacy Practice, Raghavendra Institute of Pharmaceutical Education and Research (RIPER), Anantapur, Andhra Pradesh, India.
6 Associate Professor, Department of Pharmaceutics, Raghavendra Institute of Pharmaceutical Education and Research (RIPER), Anantapur, Andhra Pradesh, India.
7 Associate Professor, Department of Pharmacology, Raghavendra Institute of Pharmaceutical Education and Research (RIPER), Anantapur, Andhra Pradesh, India.
8 Associate Professor, Department of Pharmacology, Raghavendra Institute of Pharmaceutical Education and Research (RIPER), Anantapur, Andhra Pradesh, India.
NAME, ADDRESS, E-MAIL ID OF THE CORRESPONDING AUTHOR: Dr. Bhupalam Pradeepkumar, Raghavendra Institute of Pharmaceutical Education and Research (RIPER) K R Palli Cross Chiyyedu Post, Anantapur-515721, Andhra Pradesh, India.
E-mail: pradeep@riper.ac.in
Introduction
Peltophorum pterocarpum is a rich source for phenols and the wood, leaves, and flowers of the plant are used as medicinal agents in traditional medicine. Peptic ulcer is a major disease of gastrointestinal tract, affecting nearly 40 lac people each year worldwide and affects 10% of world population with different aetiologies. Peltophorum pterocarpum consists of phenols and flavonoids, tannins which have potential antioxidant and cytoprotective activity.
Aim
To evaluate the antiulcer activity of methanolic extract of the leaves of Peltophorum pterocarpum (MEPP) on albino rats.
Materials and Methods
The rats were divided into four groups as control, standard, test 1 (extract: 100 mg/kg) and test 2 (extract: 200 mg/kg) with six rats in each group. Gastric lesions were induced by oral administration of indomethacin (20 mg/kg) followed by pylorus ligation. Standard group of animals were treated with misoprostol and test group of animals were treated with MEPP at doses of 100 and 200 mg/kg. To determine the antiulcer activity of extract, mean ulcer index, free acidity and total acidity were evaluated.
Results
Ulcer index was significantly decreased at p<0.01 in MEPP treated groups as compared to control group. Total and free acidity was significantly decreased at p<0.01 in MEPP treated groups as compared to control group. Histological analysis also supported the gastro protective effect of MEPP treated groups when compared with control groups.
Conclusion
The study revealed that MEPP exhibited potential antiulcer activity and showed dose dependent antiulcer effect.
Indomethacin, Misoprostol, Pylorus ligation, Ulcer index
Introduction
Medicinal plants have antioxidants such as phenols, carotenoids, vitamin C, tocopherols and flavonoids. Peltophorum pterocarpum is a rich source for phenols and flavonoids, its parts are used as medicinal agents in traditional medicine [1]. The leaves of the Peltophorum pterocarpum have been scientifically proven to be hepatoprotective, flowers have antibacterial property and the wood has anticancer potential [2-4].
Every year peptic ulcer affects nearly four million people worldwide and affects 10% of world population with different aetiologies [5,6]. Even today, most of the people traditionally use herbal medicine for primary health care in developing countries because of lesser side effects [7,8]. Chronic use of the most conventional antiulcer drugs may produce undesirable side effects, drug interactions and the biochemical mechanisms of the body are also affected [5,9,10].
Peltophorum pterocarpum is used for the treatment of numerous ailments like insomnia, stomatitis, constipation, skin disorders, worms and dysentery [11]. Studies have shown that Peltophorumpterocarpum is rich source for various active constituents, including flavonoids, phenolic compounds and tannins, which have medicinal uses because of their antioxidant and anti-inflammatory and curative properties and probable to have the hypothesis for antiulcer potential [6,7,11].
The aim of the present study was to evaluate the antiulcer activity of MEPP on albino rats.
Materials and Methods
A preclinical pharmacological screening for antiulcer activity was performed in Raghavendra Institute of Pharmaceutical Education and Research (RIPER), Anantapur, from November 2015 to January 2016. The protocol of this study was approved by IAEC with an approval number: 878/ac/05/CPCSEA/004/2015.
Plant Material and Extract Preparation
The leaves of Peltophorum pterocarpum were collected from the Anantapur regions, Andhra Pradesh, India. The plant material was authenticated and identified by Dr. J. Raveendra Reddy, JNTUA, Anantapur, where the voucher specimen was deposited for further reference. The leaves were dried in shade and powdered in a blender. The powder was extracted with methanol by soxhalation [2,12].
Animal Models
Albino rats of both sexes, strain Wistar, weighing 160-180 g were procured from the Raghavendra enterprises, Bangalore, Karnataka, India. The animals were housed in polypropylene cages with free access to food and water at 24±2°C, relative humidity of 40-45% and in a 12:12 hour light and dark cycle [13]. The rats were divided into four groups of six animals each, and acclimatised to laboratory atmosphere for a minimum of seven days prior to the study and were used only once throughout the experiment.
Acute Toxicity Studies
Acute toxicity test was performed on another set of albino rats and healthy male albino rats were divided into four groups of six rats in each group. The rats were fastened overnight prior to the experiment. Each group was treated orally with different doses of methanolic extract of Peltophorum pterocarpum (5 mg/kg, 50 mg/ kg, 300 mg/kg, 2000 mg/kg). The rats were observed for any gross behavioural changes and mortality as per OECD (Organization for Economic Cooperation and Development) Guidelines 425 [14]. The leaf extract of P. pterocarpum was found to be nontoxic up to the maximum dose of 2000 mg/kg body weight.
Animal Grouping and Treatment Schedule for Antiulcer Study
Albino rats weighing 160-180 g. were selected and divided into four groups of six animals each. Animals were abstained for 24 hours before the study, but had free access to water. Group I treated as control, received only distilled water; Group II and III treated as treatment groups, received with MEPP at the dose of 100 mg/ kg and 200 mg/kg respectively, and Group IV as standard group, received misoprostol 100 mcg/kg.
Gastric Lesions Induced by Indomethacin and Pylorus Ligation Model
Gastric lesions were induced to all groups of rats by oral administration of Indomethacin (20 mg/kg suspension in 1% CMC) for three successive days followed by pylorus ligation under pentobarbitone (45 mg/kg i.p.) anaesthesia on 4th day [15,16]. The standard drug misoprostol (100 mcg/kg i.p.) was administered immediately after oral indomethacin administration for three successive days. In the same manner, test compounds (MEPP dose 100 mg/kg and 200 mg/kg i.p.) were administered after oral indomethacin administration for three successive days [17].
Determination of Ulcer Index
The rats were sacrificed after four hours of pylorus ligation; abdomen was opened by making an incision. Gastric contents were collected for the determination of total acid output and the stomach was washed with water. Ulcer index was calculated with the help of a ulcer score scale.
Normal stomach without any red colouration=0
Red colouration=0.5
1 Ulcer spot=1
Haemorrhagic streaks=1.5
3-5 ulcer spots=2
More than 5 ulcer spots=3
Mean ulcer score for each group was calculated and recorded as Ulcer index [18,19].
Determination of Total Gastric Output
Gastric contents of all groups of rats were collected and centrifuged at 2000 rpm for five minutes. Supernatant clear fluid was pipetted out. A 1 ml of this fluid was mixed with 9 ml of distilled water and titrated with 0.01 N sodium hydroxide, phenolphthalein being used as an indicator. Titre value was recorded at the end point (Pink to orange colour) which was expressed as free acid. Titration was continued till the reappearance of pink colour and the titre value was recorded again which was expressed as total acid [16].
Histological Analysis
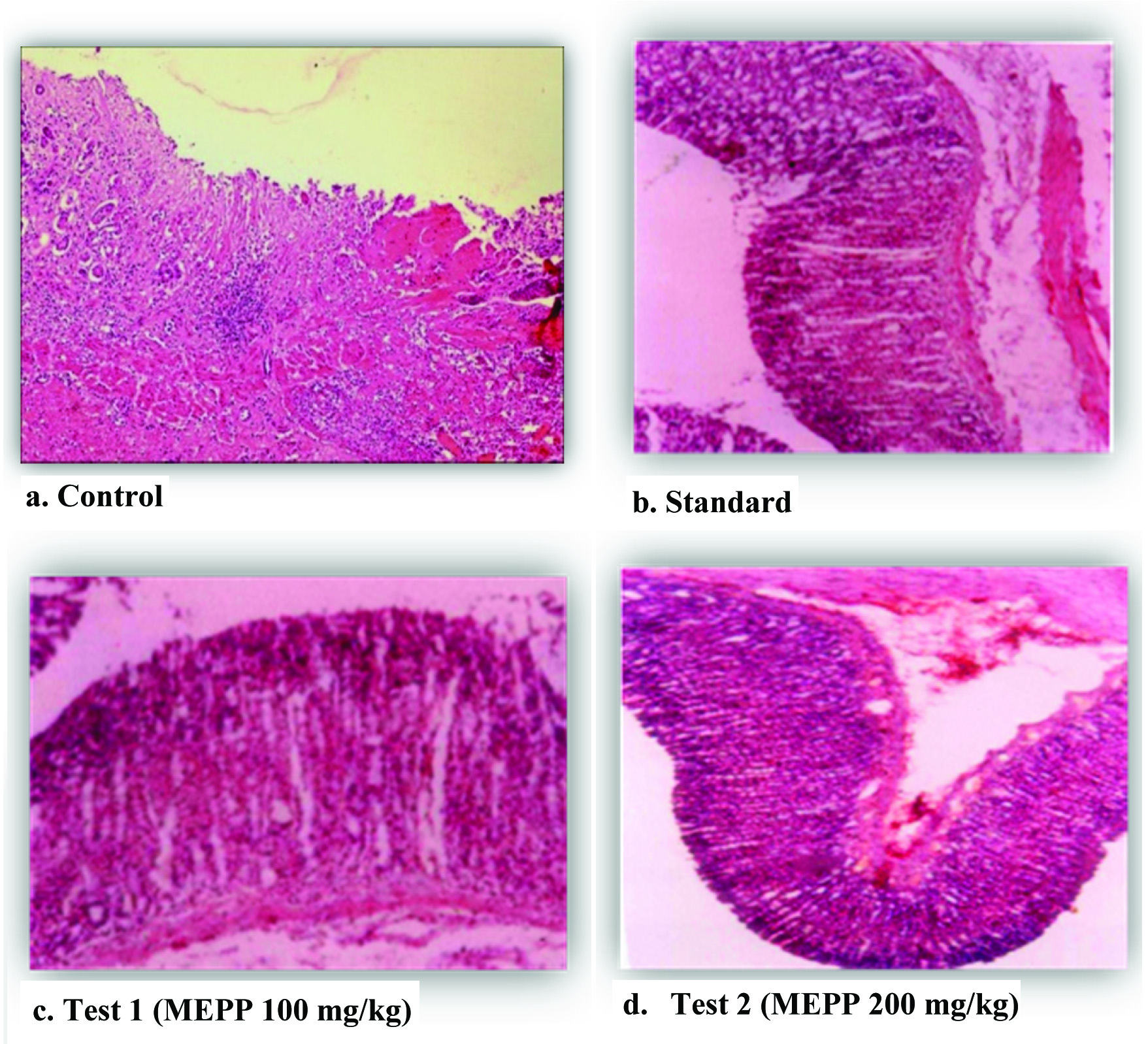
The stomachs of all groups of rats were preserved in a 10% buffered formalin solution and processed for subsequent embedment. The tissue samples were cut into sections of 2.5 µm. Two slides were prepared and stained with Haematoxylin–Eosin (HE) and Periodic Acid-Schiff (PAS) [20]. The slides were appraised by light microscopy (10X).
Statistical Analysis
The results were expressed as the mean±SEM. Statistical differences were evaluated using a one-way analysis of variance (ANOVA) followed by Dunnett’s t-test. Results were considered to be statistically significant at p<0.05.
Results
The animals treated with MEPP 100 mg/kg and 200 mg/kg showed significant as well as dose dependent inhibition (p<0.01) of ulcer index when compared with control group [Table/Fig-1]. Moreover, free acidity and total acidity also significantly decreased (p<0.01) in the animals treated with MEPP 100 mg/kg and 200 mg/kg when compared with control group [Table/Fig-2]. Additionally, histological analysis also supported that the treatment with MEPP showed gastroprotection with both doses against indomethacin induced gastric damage when compared with control group [Table/Fig-3].
Effect of MEPP on ulcer index.
| Treatment | Ulcer index (Mean±SEM) |
|---|
| Control (Vehicle) | 4.16±0.47 |
| Standard (Misoprostol) | 0.25±0.11** |
| Test 1 (MEPP: 100 mg/kg) | 1.91±0.15** |
| Test 2 (MEPP: 200 mg/kg) | 1.08±0.2** |
Values are expressed as Mean±SEM, n= 6,
p<0.01 when compared with control group. (Statistically analysed by one-way ANOVA followed by Dunnett’s t-test).
Effect of MEPP on free acidity and total acidity.
| Treatment | Free acidity | Total acidity |
|---|
| Control (Vehicle) | 46.3±0.76 | 52.83±0.30 |
| Standard (Misoprostol) | 11.16±0.47** | 19.5±0.42** |
| Test 1 (MEPP: 100 mg/ kg) | 22.16±0.30** | 30.5±0.42** |
| Test 2 (MEPP: 200 mg/ kg) | 15.83±0.30** | 24.5±0.42** |
Values are expressed as Mean±SEM, n= 6,
p<0.01 when compared with control group. (Statistically analysed by one-way ANOVA followed by Dunnett’s t-test).
Histopathological analysis (10X): (Haematoxylin–Eosin and Periodic Acid-Schiff).
Discussion
Greater beneficial effects have been reported for natural products in folk medicine. Studies on medicinal plants, have shown them to be alternative source for new compounds with potential pharmacological activity [6]. Gastroprotective effect of MEPP was evaluated against indomethacin induced ulcers in rat model. NSAIDs were known to cause ulcer by inhibiting prostaglandin synthesis. Prostaglandin synthesis is inhibited by Cyclooxygenase (COX) enzyme inhibitors like indomethacin and induces gastric damage [20,21].
The results of this study revealed that the MEPP was beneficial to treat gastric ulcers by preventing oxidative stress. The mechanism of gastro protection of Peltophorum pterocarpum by antioxidant effect may be mediated by stimulation of prostaglandin biosynthesis [6,20,21]. Prostaglandin synthesis protects the stomach from the irritation and injuries by stimulating the secretion of protective substance mucus and bicarbonates [22]. The MEPP reduces mucosal damage compared to control. The ability of the extract to reduce gastric mucosal damage further supports cytoprotective effect and suggests the possible involvement of prostaglandins in the antiulcer effect of the extract [22]. Acute toxicity studies showed no toxic symptoms at 2000 mg/kg, which indicates that MEPP was safe. However subacute and carcinogenic studies have to be performed before clinical studies.
Further detailed study on the isolation and characterisation of the compounds responsible for the antiulcer activity in methanolic extract of Peltophorum pterocarpum needs to be carried out.
Conclusion
The results obtained in this study showed that the methanolic extract of Peltophorum pterocarpum possesses antiulcer activity and showed the dose dependent gastroprotective effect against indomethacin induced and pylorus ligation ulcers.
Values are expressed as Mean±SEM, n= 6,
**p<0.01 when compared with control group. (Statistically analysed by one-way ANOVA followed by Dunnett’s t-test).
Values are expressed as Mean±SEM, n= 6,
**p<0.01 when compared with control group. (Statistically analysed by one-way ANOVA followed by Dunnett’s t-test).
[1]. Khan M, Rizwani GH, Shareef H, Cavar S, Zia-Ul-Haq M, Assessment of total phenolic content and antioxidant potential of methanol extract of Peltophorum pterocarpum (DC.). Backer ex K. HeynePak J Pharm Sci 2013 26(5):967-72. [Google Scholar]
[2]. Biswas K, Kumar A, Babaria BA, Prabhu K, Setty RS, Hepatoprotective effect of leaves of Peltophorum pterocarpum against paracetamol Induced acute liver damage in ratsJournal of basic and clinical pharmacy 2009 1(1):10-15. [Google Scholar]
[3]. Sukumaran S, Kiruba S, Mahesh M, Nisha SR, Miller PZ, Ben CP, Phytochemical constituents and antibacterial efficacy of the flowers of Peltophorum pterocarpum (DC.). Baker ex HeyneAsian Pacific journal of tropical medicine 2011 4(9):735-38. [Google Scholar]
[4]. Zhang J, Nishimoto Y, Tokuda H, Suzuki N, Yasukawa K, Kitdamrongtham W, Cancer chemopreventive effect of bergenin from Peltophorum pterocarpum woodChemistry & biodiversity 2013 10(10):1866-75. [Google Scholar]
[5]. Sahoo SK, Sahoo HB, Priyadarshini D, Soundarya G, Kumar CK, Rani KU, Antiulcer Activity of Ethanolic Extract of Salvadora indica (W.) Leaves on Albino RatsJ Clin Diagn Res 2016 10(9):Ff07-ff10. [Google Scholar]
[6]. Bento EB, Júnior FEdB, de Oliveira DR, Fernandes CN, de Araújo Delmondes G, Cesário FRAS, Antiulcerogenic activity of the hydroalcoholic extract of leaves of Annona muricata Linnaeus in miceSaudi Journal of Biological Sciences 2016 [Google Scholar]
[7]. Vimala G, Gricilda Shoba F, A review on antiulcer activity of few Indian medicinal plantsInternational journal of microbiology 2014 2014:519590 [Google Scholar]
[8]. Nagulsamy P, Ponnusamy R, Thangaraj P, Evaluation of antioxidant, anti-inflammatory, and antiulcer properties of vaccinium leschenaultii Wight: A therapeutic supplementJournal of Food and Drug Analysis 2015 23(3):376-86. [Google Scholar]
[9]. Vasudeva N, Sethi P, Sharma SK, Kumar S, Sharma S, Antiulcer potential of the ethanolic extract of aerva persica merrill root in ratsJournal of Acupuncture and Meridian Studies 2012 5(2):80-86. [Google Scholar]
[10]. Mohod SM, Bodhankar SL, Antiulcer activity of aqueous extract of leaves of Madhuca indica J. F. Gmel against naproxen induced gastric mucosal injury in ratsJournal of Acute Disease 2013 2(2):127-33. [Google Scholar]
[11]. Jash KS, Singh KR, Majhi S, Sarkar A, Gorai D, Peltophorum pterocarpum: Chemical and pharmacological aspectsInternational journal of pharmaceutical sciences and research 2014 5(1):26-36. [Google Scholar]
[12]. Veerabhadrappa KV, Pradeep Kumar B, Sudheer A, Haranath C, Somasekharreddy Synergistic antidiabetic activity of liquorice and jatamansi in alloxan induced diabetic ratsInternational Journal of Current Research 2015 7(6):16867-72. [Google Scholar]
[13]. CPCSEACPCSEA Guidelines for lab facility 2003Indian J Pharmacol 2003 35:257-74. [Google Scholar]
[14]. OECD Test Guideline 425. Guidelines for Testing of ChemicalsGuidelines 425, Acute Oral Toxicity-Up-and-Down Procedure 2001 [Google Scholar]
[15]. Baiubon P, Kunanusorn P, Khonsung P, Chiranthanut N, Panthong A, Rujjanawate Gastroprotective activity of the rhizome ethanol extract of Zingiber simaoense Y. Qian in ratsJournal of Ethnopharmacology 2016 194:571-76. [Google Scholar]
[16]. Pradeepkumar B, Reddy P, Devanna N, Somasekhar Reddy K, Sudheer A, Naresh Babu G, Evaluation of anti ulcer effect of polyalthia longifolia leaves in albino ratsInrernational journal of chemistry and pharmaceutical sciences 2015 3(3):1584-86. [Google Scholar]
[17]. Gupta JK, Neeraj U, Patnaik AK, Mitra PM, Evaluation antiulcer activity of leucas lavandulifolia onmucosal lesion in ratAsian J of Pharmaceuticaland Clinical Res 2010 3(2):118-20. [Google Scholar]
[18]. Li XM, Miao Y, Su QY, Yao JC, Li HH, Zhang GM, Gastroprotective effects of arctigenin of Arctium lappa L. on a rat model of gastric ulcersBiomedical reports 2016 5(5):589-94. [Google Scholar]
[19]. Agrawal R, Garg HK, Garg U, Singh SK, Anti-ulcer activity of Smithia conferta in various animalJournal of Saudi Chemical Society 2010 14(3):307-10. [Google Scholar]
[20]. Pereira AC, Lenz D, Nogueira BV, Scherer R, Andrade TU, Costa HB, Gastroprotective activity of the resin from Virola oleiferaPharmaceutical biology 2017 55(1):472-80. [Google Scholar]
[21]. Suleyman H, Albayrak A, Bilici M, Cadirci E, Halici Z, Different mechanisms in formation and prevention of indomethacin-induced gastric ulcersInflammation 2010 33(4):224-34. [Google Scholar]
[22]. Sofidiya MO, Awolesi AO, Antinociceptive and antiulcer activities of Pycnanthus angolensisRevista Brasileira de Farmacognosia 2015 25(3):252-57. [Google Scholar]