Oral cancer is a heterogeneous disease that arises in various subsites of the oral cavity with different risk factors, prevalence and outcomes [1]. In India, oral cancer was one of the leading cancers; where an estimated 83,000 new cases and 46,000 oral cancer related deaths occur yearly [2]. In South India, the most common subsite is buccal mucosa due to frequently keeping tobacco quid against the buccal mucosa at vestibule [3].
Oral mucosal lesions can be due to infection (bacterial, viral, fungal), local trauma and/or irritation (traumatic keratoses, fibroma, burns), systemic diseases (metabolic or immunologic), or related to lifestyle factors such as the usage of tobacco, areca nut, betal quid, or alcohol [4]. Although several factors are related to oral cancer, the primary risk factor is tobacco chewing and smoking habits. Overall, 95% of oral cancer is caused by tobacco consumption [5]. The leading risk habit is betal nut chewing; it is the fourth most commonly used psychoactive substance in the world after caffeine, alcohol and nicotine [6]. Pan masala chewing consists areca nut with slaked lime; catechu and other flavouring agents and alcohol consumption habit has become a part of lifestyle among young adults in many rural and urban areas. Recently, non tobacco habits showed high incidence of oral cancer [7-9].
Despite advances in cancer treatment, the survival rates of oral cancer have not improved substantially [5]. Aetiology is proposed as one of the significant prognostics factor with adverse effect on survival in any form of tobacco (chewing or smoking) as well as non tobacco (pan masala) [10]. Therefore, there is need to understand the risk of aetiology in pathogenesis of the diseases. Hence, the aim of this study was to analyse RR of tobacco and non tobacco users in buccal mucosa carcinoma.
Materials and Methods
This prospective comparative study was carried out in Department of Oral Oncology, Arignar Anna Memorial Regional Cancer Centre and Research Institute, Kanchipuram, Tamil Nadu, between March 2013 and January 2016. The Institutional Ethical Clearance was obtained to conduct the study (Ref No. 24984/2013). A written informed consent was taken from the patients.
A total of 198 buccal mucosa carcinoma subjects were assessed between 2013 and 2014 and finally 117 (59.1%) buccal mucosa carcinoma subjects who had habits of consuming either tobacco or non tobacco were included in the study. Precancerous and multihabitual (tobacco and non tobacco consumption) buccal mucosa carcinoma subjects were excluded from the study.
The subjects were divided as two groups according to the habits; Group-I included smoking and smokeless tobacco and Group II included areca nut, betal quid without tobacco, pan masala and alcohol.
Data collection: A self prepared questionnaire was used to collect the demographic characteristics of age, gender, marital status, Body Mass Index (BMI), occupation, education, income and their habits (tobacco chewing, smoking, pan, areca nut chewing and alcohol) and clinical features like clinical TM stage, nodal and metastasis status and degree of differentiation from medical records.
The subjects were followed-up for every three months, after treatment for three years. The total duration of follow up was two years.
Statistical Analysis
The results were expressed as numbers and percentages. Descriptive statistics were calculated using Chi-square analysis or Fisher’s exact test. Overall survival was calculated as the time from the first date of treatment to the date of death or last known date the patient was alive. The survival rate was estimated by Kaplan-Meier analysis and log-rank test was used to compare the survival of tobacco and non tobacco users. The results of the null hypothesis were less than or equal to 0.05.
Results
Demographic profile: The present study included 117 buccal mucosa carcinoma subjects (87 tobacco users and 30 non tobacco users in the proportion of 3:1). [Table/Fig 1] shows the association of demographic characteristics according to tobacco and non tobacco users. Of 117 subjects, 61 (52.1%) male and 56 (47.9%) female subjects had significant risk habit difference (p=0.023, p<0.05) with 0.365 (95CI, 0.150-0.886) fold risk. Majority of subjects were in old age group groups 59 (50.4%) with mean age of 52.2 years (Range: 24-88 years). The present study shows significant association between young (<40 years) and older age (≥40 years) groups (p=0.04, p<0.05). Further, the study revealed tobacco users had 2.026 (95CI, 0.905-4.996) fold risk of developing disease than non tobacco users. The rest of characteristics of marital status, BMI and socioeconomic status did not show significant difference based on tobacco and non tobacco usage by Chi-square crude RR analysis.
Demographic characteristics and its association with tobacco and non-tobacco habitual subjects.
| Characteristics | Tobacco (n=87) | Non tobacco (n=30) | Risk ratio (95%CI) | p-value |
|---|
| Gender |
| Male | 40 (46) | 21(70) | 0.365 (0.1500.886) | 0.023* |
| Female | 47 (54) | 9 (30) |
| Marital status |
| Married | 57 (65.5) | 20 (66.7) | 0.902 (0.4151.958) | 0.909 |
| Single/divorced/widow | 30 (34.5) | 10 (33.3) |
| Age (years) |
| ≥40 | 39 (44.8) | 19 (63.3) | 2.026 (0.9054.996) | 0.04* |
| >40 | 48 (55.2) | 11 (36.7) |
| BMI |
| Underweight (<18.5 kg/m2) | 35 (40.2) | 9 (30) | - | 0.737 |
| Healthy weight (18.5-24.9 kg/m2) | 23 (26.4) | 10 (33.3) |
| Overweight (25-29.9 kg/m2) | 19 (21.8) | 8 (26.7) |
| Obese (30-35 kg/m2) | 10 (11.5) | 3 (10) |
| SES |
| Upper | 3 (3.4) | 2 (6.7) | - | 0.129 |
| Upper middle | 8 (9.2) | 6 (20) |
| Lower middle | 15 (17.2) | 2 (6.7) |
| Lower upper | 21 (24.1) | 3 (10) |
| Lower | 40 (46) | 17 (56.7) |
BMI- Body Mass Index; SES-Socioeconomic status;
Significant by chi-square analysis at p<0.05 level
Clinical information: [Table/Fig-2] shows the relation of aetiology and clinical characteristics of subjects. The clinical features of clinical TNM stage (p=0.024), nodal status (p=0.017), tumour cell differentiation (p=0.015), perineural invasion (p=0.012) and extracapsular invasion (p=0.045) had shown significantly different characteristics in tobacco users and non tobacco users. The present study consisted of more tobacco user’s, the clinical characteristics of clinical stage 1.57 fold (95% CI, 0.338-3.31), nodal status 2.014 fold (95% CI, 0.412-4.454), tumour cell differentiation 1.293 fold (95% CI, 0.581-2.878), perineural invasion 2.601 fold (95% CI, 0.806-5.32) and extracapsular invasion 1.627 fold (95% CI, 0.533-2.824) increased risk of development of buccal mucosa carcinoma than non tobacco users.
Clinical characteristics and its association with tobacco and non-tobacco habitual subjects.
| Characteristics | Tobacco (n=87) | Non tobacco (n=30) | Risk ratio | p-value |
|---|
| (95%CI) |
|---|
| Clinical TNM Stage |
| Early stage (I and II) | 15 (17.2) | 5 (16.7) | 1.57 (0.338-3.31) | 0.024* |
| Advanced stage (III and IV) | 72 (82.8) | 25 (83.3) |
| Tumour class |
| T1 and T2 | 27 (31.3) | 12 (40) | 0.306 (0.066-1.42) | 0.13 |
| T3 and T4 | 60 (68.7) | 18 (60) |
| Nodal status |
| Negative (N0) | 15 (17.2) | 4 (13.3) | 2.014 (0.412-4.454) | 0.017* |
| Positive (N1,N2,N3) | 72 (82.8) | 26 (86.7) |
| Metastasis status |
| Negative (M0) | 66 (75.9) | 20 (66.7) | 1.575 (0.636-3.881) | 0.325 |
| Positive (M1) | 21 (24.1) | 10 (33.3) |
| Grade of tumour |
| Well differentiated | 34 (39.1) | 14 (46.7) | 1.293 (0.581-2.878) | 0.015* |
| Moderately differentiated | 39 (44.8) | 7 (23.3) |
| poorly differentiated | 14 (16.1) | 9 (30) |
| Lymphovascular Invasion |
| Negative (-) | 28 (32.2) | 9 (30) | 1.107 (0.450-2.727) | 0.824 |
| Positive (+) | 59 (67.8) | 21 (70) |
| Perineural Invasion |
| Negative (-) | 27 (31) | 6 (20) | 2.601 (0.806-5.32) | 0.012* |
| Positive (+) | 60 (69) | 24 (80) |
| Pattern of Invasion |
| Type I | 14 (16.1) | 7 (23.3) | - | 0.643 |
| Type II | 26 (29.9) | 9 (30) |
| Type III | 47 (54) | 14 (46.7) |
| Extracapsular Invasion |
| None | 57 (65.5) | 21 (70) | 1.627 (0.533-2.824) | 0.045* |
| Moderate | 16 (18.4) | 5 (16.7) |
| High | 14 (16.1) | 4 (13.3) |
Significant by chi-square analysis at p<0.05 level.
Rest of the clinical features metastasis status, lymphovascular invasion and pattern of invasion had failed to show difference based on habits of individuals.
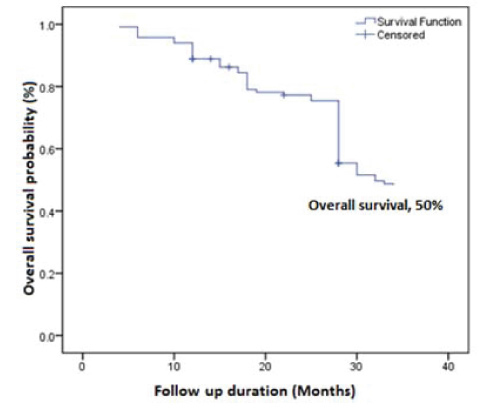
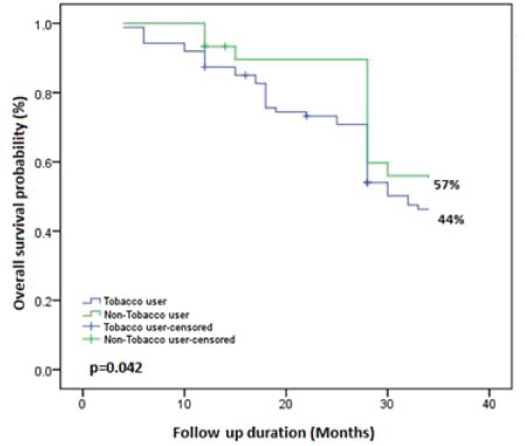
Analysis of prognostic factors: A total period of follow up was 34 months (median, 24 months); whoever missing last follow up, contacted through phone. Overall, at the last follow up, two (1.7%) subjects had died, four (3.4%) subjects were identified with recurrence and 111 (94.9%) subjects were alive without any evidence of the disease [Table/Fig-3]. [Table/Fig-4] showed overall survival of buccal mucosa carcinoma subjects (50%) by Kaplan-Meier survival analysis [Table/Fig-5] revealed that tobacco users, smokers and/or chewers (44%) had a significantly poorer survival when compared to the non tobacco users (57%) by Kaplain-Meier survival analysis using log-rank test (p=0.042, p<0.05).
Overall survival difference between tobacco and non tobacco users.
| Habits | Total no. of subjects (n=117) | No. of DSD (n=2) | no. of recurrent (n=4) | Survival rate | p-value |
|---|
| Tobacco users | 87 (74.3) | 2 (100) | 3 (75) | 44 | 0.042* |
| Non tobacco users | 30 (25.7) | 0 | 1 (25) | 57 |
DSD= Disease Specific Death
Significant at p<0.05 level by Kaplan-Meier survival analysis using log-rank test
Overall survival of buccal mucosa carcinoma by Kaplan-Meier analysis.
Overall survival difference of tobacco users and non tobacco users by Kaplan-Meier (Log-rank test) analysis.
Discussion
There is a strong association between the consumption of tobacco and non tobacco habits, incidence of oral cancer and reduction of their consumption with the prevention of cancer [11]. Tobacco is used in a variety of ways, mostly smoked, but many populations use smokeless tobacco, which comes in two main forms; snuff (finely ground or cut tobacco leaves that can be dry or moist, loose or portion packed in sachets) and chewing tobacco (loose leaf, in pouches of tobacco leaves, plug or twist form). Two main carcinogens present in tobacco smoke are benzo/pyrine derived nitrosamines [12]. Areca nut contains various alkaloids (arecoline, arecaidine, guvacine, guvacoline) which lead to formation of nitrosamines in the saliva that are carcinogenic and addictive substances [8,9,13].
The use of betel quid containing both areca nut and tobacco is associated with 8-15 times higher RR of oral cancer compared to the use of betal quid without tobacco and proves the aggressiveness of tobacco in oral cancer pathogenesis [14]. Further, a study from Northern Italy found that smoking is an independent risk factor which accounted for 42-47% oral cancers in females and 81-87% of oral cancers in males [15]. The reported pooled cancer risk estimate is 3.43 times higher in smokers compared with non smokers (95% CI 2.37-4.94) [16]. In accordance to the previous reports, the present study showed the RR of demographic and clinical characteristics among tobacco and non tobacco habitual buccal mucosa carcinoma subjects.
The Indian studies report male preponderance and is largely attributed to the increasing use of tobacco and non tobacco habits [17-19]. A cohort study conducted in Mumbai found relative risk difference of smoking tobacco, smokeless tobacco and non tobacco habits among gender basis. The study revealed, the RR of smoking in men to be 1.37 (95% CI, 1.23–1.53) fold than women. Similarly, women showed RR of smokeless tobacco was 1.25 (95% CI, 1.15–1.35) [20]. However, the present study shows the significant risk difference among gender.
Most of the Indian studies reported occurrence of oral squamous cell carcinoma in the fifth decade of life [4,5]. In contrary, a South Indian study showed increased incidence of buccal cancers among younger patients <35 years [21]. A recent study based on tobacco use in younger and old patients of tongue squamous cell carcinoma found insignificant difference of tobacco role in younger (<40 years) and elder (≥40 years) age groups of patients [22]. The present study showed the high incidence of older than younger patients with mean age of 53 years. In this study most of younger patients had habits of non tobacco chewing and old patients had a habit of tobacco chewing and smoking. However, the present study revealed that aetiology had significant difference in age groups; tobacco users had 2.026 fold of increased RR of occurrence than non tobacco users.
The aetiology of oral cancer has geographic variation in occurrence, prevention and outcome [23]. Schmidt et al., revealed in his study that oral squamous cell carcinoma was more aggressive (poorly differentiated) in tobacco users than non tobacco users [17]. In contrary, oral cancer did not find significant association of histological grading and smoking habits [24]. However, the present study is coherent with previous results, tobacco habitual showed 1.293 fold RR of aggressive poorly differentiated risk than non tobacco habitual. Additionally, presence of perineural and extracapsular invasion pathological features also had 2.601 and 1.627 fold risk of development of disease in tobacco habitual.
In clinical routine, TNM staging of tumours had been used as important tool for further treatment. Krishnamurthy A and Ramshanker V found with early Stage I and II of tongue squamous cell carcinoma and association with non tobacco habits [22]. In contrary, the present study found with high frequency of tobacco chewers and significant difference with 1.57 fold risks of stage of tumour and additional co-factor of nodal status also showed 2.014 fold RR of occurrence in tobacco than non tobacco chewers. Further, wide study on non tobacco risk of buccal mucosa carcinoma warranted to evaluate the risk of disease.
Limitation
The present study shows the RR (demographic and clinical features) of tobacco and non tobacco habitual users in a single institute with limited number of patients. Further, population wide study is warranted to evaluate the RR analysis.
Conclusion
The present study illustrates that aetiology and its association with demographic-clinical factors, might help to improve prognosis. The study revealed aggressiveness of tobacco with poor survival rate than non tobacco users. Hence, the present study suggests nationwide counselling on prevention of tobacco used in India.
BMI- Body Mass Index; SES-Socioeconomic status;
*Significant by chi-square analysis at p<0.05 level
*Significant by chi-square analysis at p<0.05 level.
DSD= Disease Specific Death
*Significant at p<0.05 level by Kaplan-Meier survival analysis using log-rank test