Improved Serodiagnostic Sensitivity of Strip Test for Latent Tuberculosis
Songsri Kasempimolporn1, Wichit Thaveekarn2, Kanyanat Promrungreang3, Orawan Khow4, Supatsorn Boonchang5, Visith Sitprija6
1 Senior Advisory Scientist, Department of Research and Development, Queen Saovabha Memorial Institute, Thai Red Cross Society, Bangkok, Thailand.
2 Scientist, Department of Research and Development, Queen Saovabha Memorial Institute, Thai Red Cross Society, Bangkok, Thailand.
3 Scientist, Department of Research and Development, Queen Saovabha Memorial Institute, Thai Red Cross Society, Bangkok, Thailand.
4 Senior Scientist, Department of Research and Development, Queen Saovabha Memorial Institute, Thai Red Cross Society, Bangkok, Thailand.
5 Laboratory Officer, Department of Research and Development, Queen Saovabha Memorial Institute, Thai Red Cross Society, Bangkok, Thailand.
6 Director Professor, Queen Saovabha Memorial Institute, Thai Red Cross Society, Bangkok, Thailand.
NAME, ADDRESS, E-MAIL ID OF THE CORRESPONDING AUTHOR: Dr. Songsri Kasempimolporn, 1871 Rama IV Road, Bangkok, Pathumwan, Thailand.
E-mail: songsri.k@redcross.or.th
Introduction
Diagnosis of Latent Tuberculosis Infection (LTBI) is difficult due to no clinical manifestations. Cases of LTBI are mostly sputum negative. The World Health Organization recommends the Tuberculin Skin Test (TST) as the current diagnostic standard for LTBI. Our previously developed serologic strip test for LTBI detection had suboptimal sensitivity. Additional Mycobacteriumtuberculosis (MTB) latency-associated antigens may improve the detection rate of LTBI.
Aim
The present study aimed to optimize sensitivity of existing strip test.
Materials and Methods
A combination of recombinant latency proteins Rv2029c, Rv2031c, Rv2032, Rv2627c, Rv3133c, and Rv3716c was used to prepare the strips and evaluate the performance with the sera of patients in four well-classified categories: LTBI, active pulmonary TB, healthy TB contacts and other non-TB diseases.
Results
A total of 91 serum samples from various clinical categories were screened with the strips. Among clinically diagnosed LTBI patients, strip test yielded a sensitivity of 75.0%. Among clinically diagnosed non-LTBI subjects, strip test yielded 88.1% specificity. The diagnostic positive and negative predictive values for strip test in reference to various clinical contexts were 77.4% and 86.7%, respectively.
Conclusion
Addition of the six potential latency proteins could improve the diagnostic performance of existing strip test for LTBI. The use of suitable immunodominant antigens could maximize sensitivity in the diagnosis and differentiate MTB infection status.
Immunochromatographic test, Latency antigen, Latent tuberculosis infection
Introduction
Tuberculosis (TB) is a major threat to global health. The WHO ‘End TB Strategy’ aims to end the global TB epidemic by 2035 [1], with targets to afford sensitive diagnostic tests for all forms of TB and a vaccine that can prevent people with latent TB from progressing to active disease. It has been estimated that one-third of the world’s population is latently infected with MTB and approximately 10% of individuals with LTBI will develop active disease during lifetime [2]. The enormous reservoir of LTBI poses a major hurdle for global TB control. Early detection of LTBI will allow appropriate treatment and will aid disease control. LTBI is defined by the presence of a MTB-specific immune response in the absence of clinical and radiological disease [3]. The Tuberculin Skin Test (TST) or an Interferon-Gamma Release Assay (IGRA) is currently the best method to identify those with LTBI. TST is considered the gold standard, despite some limitations. The requirement for TST reading after 48-72 hours increases loss to follow-up. The TST also raised concern about the low specificity of assay [4]. IGRA could be more helpful but are more costly and technically complex to do than the TST. Replacing the TST by IGRA as a public health intervention in resource-constrained settings is not recommended by WHO. In addition, IGRA do not discriminate between LTBI and active disease and are therefore not recommended in high burden settings due to the large proportion of TB cases in those settings [5]. Different types of blood test have been suggested for diagnosis of active TB disease, but all are bad practice [6]. The sensitivity of serological assays has been disappointing and appears not to be useful as a sole diagnostic tool for active TB in comparison with the current diagnostic standards. However, blood tests for LTBI have not been evaluated because data on patients with LTBI are limited. We previously had taken an immunochromatographic approach based on MTB antigens which associated both with LTBI and active TB disease, and found suboptimal sensitivity [7]. Further improvements are needed before this rapid test could be useful for clear-cut diagnosis of LTBI. The study of antigen candidates that may be associated only with LTBI is a priority to enhance the sensitivity of diagnosis. An interesting approach toward latency antigens is their association with humoral immunity. For optimize sensitivity, MTB latency-associated antigens have been chosen from dormancy survival regulon (DosR) [8] and used as diagnostic antigens for the preparation of test strips. The detection of antibodies against these antigens in the sera obtained from four well-classified categories of patients was evaluated.
Materials and Methods
It was an experimental study. The sera used in the present study were obtained in the previous investigation [7] which was stored at -20ºC. All serum samples were collected from patients during the period between October 2012 and September 2013 in an age group of 1-58 years with a median of nine year that belonged to four well-defined categories at Phramongkutklao Hospital in Bangkok. In all cases, diagnosis was confirmed by microscopic examination of the sputum, chest radiography, IGRA (QuantiFERON®-TB Gold In Tube test, Cellestis, Carnegie, Australia) and TST (purified protein derivative RT23, Statens Serum Institute, Copenhagen, Denmark). Category1 (n=12) comprised patients with definite active pulmonary TB. Category 2 (n=32) comprised patients with LTBI who were with both positive TST and IGRA or IGRA alone, free of TB symptoms and radiographic evidence of current disease. Category 3 (n=14) comprised patients who were household TB contacts with negative IGRA and without clinical evidence of TB. Category 4 (n=33) comprised patients with other non-TB diseases, such as Non-Tuberculous Mycobacteria (NTM) lymphadenitis, bronchitis, cancer and pneumonia. A blood sample was collected followed by the TST.
Expression and purification of the recombinant proteins:
A total of six MTB latency proteins Rv2029c, Rv2031c, Rv2032, Rv2627c, Rv3133c, and Rv3716c [9-12] were selected as candidate antigens for the detection of specific antibodies in sera of various clinical categories. The latency-associated genes were PCR amplified from the genomic DNA of MTB H37Rv. All proteins were expressed as recombinant products in Escherichia coli and purified by column chromatography [13].
Strip test
The test strip was prepared in-house by following the preparation processes described in our previous work [7]. Briefly, a combination of six recombinant protein antigens was conjugated with the colloidal gold as the detector reagent. Staphylococcal protein A and rabbit anti-MTB immunoglobulin were immobilized on the nitrocellulose membrane at the test line and at the control line, respectively. Interpretation of the results was completed in 15 minute after serum sample application. The investigator who performed the strip test was blinded to the status of all samples. To evaluate the antibody responses directed against the selected antigens on strips, sera from the cases of LTBI patients were used to test for sensitivity, as well as the cases of active TB, healthy TB contacts and other non-TB diseases were used to test for specificity.
Statistical Analysis
Sensitivity, specificity, positive and negative predictive values were calculated to evaluate the performance of strip test in reference to clinical diagnosis of patients.
Results
The selected MTB latency related proteins Rv2029c, Rv2031c, Rv2032, Rv2627c, Rv3133c, and Rv3716c were expressed and displayed major antigenic stretches. SDS-PAGE revealed the molecular weight and purity of the proteins [Table/Fig-1]. A combination of these proteins was used as diagnostic antigens for the preparation of strips to increase diagnostic performance.
SDS-PAGE of purified MTB latency proteins. M, molecular weight marker (kDa).
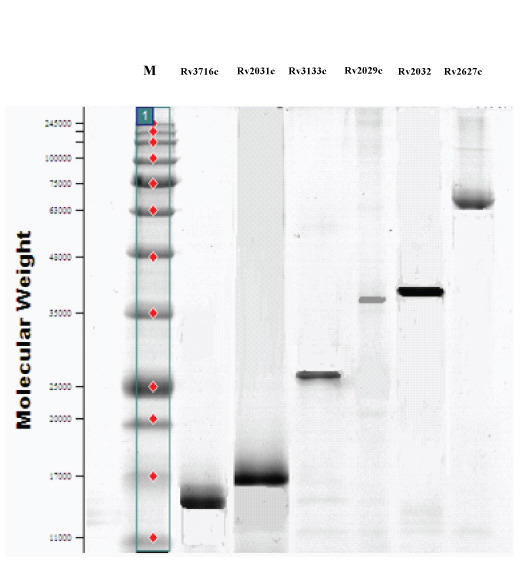
Of the 32 patients with LTBI, results of strip test were positive for 24 serum samples yielding a sensitivity of 75.0%. Among the 59 non-LTBI subjects, 52 were stripping negative yielding a specificity of 88.1% [Table/Fig-2]. Of the 12 TB patient serum samples, 4 (33.3%) were positive by strip test. None of the 14 healthy TB contacts had a positive strip test result. Of the 33 serum samples from the cases of other diseases, strip test identified three as positive (9.1%) [Table/Fig-3]. The diagnostic positive and negative predictive values for strip test in reference to clinical contexts were 77.4% and 86.7%, respectively [Table/Fig-2].
Results of strip test by testing sera of different clinical categories in reference to clinical context of LTBI.
| Strip test | Patients belonged to LTBI category | Total |
|---|
| Yes | No |
|---|
| PositiveNegative | 248 | 752 | 3160 |
| Total | 32 | 59 | 91 |
Sensitivity = 75.0% (24/32) Specificity = 88.1% (52/59) Positive predictive value = 77.4% (24/31) Negative predictive value = 86.7% (52/60)
Strip test results on four well-classified categories of patients.
| Patient group | Median age (y) | No. of case | Positive rate |
|---|
| No. | % |
|---|
| Latent TB | 12 | 32 | 24 | 75.0 |
| Active TBHealthy TB contactsNon-TB diseases | 167.510 | 121433 | 403 | 33.309.1 |
Discussion
None of the laboratory tests (i.e., smear, culture, nucleic acid amplification tests) for active TB work for LTBI. For LTBI, the diagnostic tests have always been immune-based, including tests of cellular immune response (TST and IGRA) and serological assays based on humoral immune response. A diagnostic test that can distinguish LTBI from active TB is essential in routine clinical practice to control TB and provide effective treatment for LTBI. Currently, diagnosis of LTBI relies on the TST, a century-old test with known limitations. The test was unable to distinguish between active TB disease and LTBI. TST is prone to both false-negative and false-positive results. Host factors, such as young age, poor nutrition, immunosuppression, viral infections can decrease TST reactivity [14]. A major drawback of the TST is its low specificity due to the use of tuberculin, a mixture of more than 200 nonspecific antigens shared with several NTM and with all BCG strains [15]. BCG vaccination at birth and infection with NTM are more likely to have a negative impact on TST results. The specificity of TST in our previous study was 68.1% [7]. The lower specificity of TST is associated with a high number needed to treat value. Many attempts have been made to identify and isolate specific antigens from MTB that are absent in BCG and most NTM for use as diagnostic reagents. Several antigens have been tested for use in serodiagnosis but no test with a single antigen has proved to achieve sensitivity and specificity [16,17]. Antibody responses are directed against a broad set of antigens and responses vary between individuals [18]. Adding multiple antigens that are unique to MTB in a diagnostic test to cover the broad spectrum of antigen recognition by different individuals may increase the sensitivity of tests. The targets of the immune response during LTBI differed from those occurring during active disease [19]. A set of at least 48 genes belonging to the DosR are known as latency antigens [8]. The previous studies of serological responses to a DosR antigen Rv2031c suggest that humoral responses to this antigen are minimal during active disease, while chronically exposed but healthy individuals who may have been latently infected had high titers of antibody against this antigen [20,21]. Human antibody and T-cell responses against DosR-encoded antigens in LTBI have been reported by some studies, however, the results are somewhat varied [22-26]. Of those studies, some novel DosR-encoded proteins were identified as the most frequently recognized antigens in latently infected individuals. Six of which, including Rv2029c, Rv2031c, Rv2032, Rv2627c, Rv3133c, and Rv3716c, were therefore selected as LTBI diagnostic candidates in the present study.
From data on sensitivity and specificity reported in this study, use of a combination of MTB latency antigens in the strip test improved the sensitivity while still maintaining a high specificity as known from prior work [7]. Based on the results in this study, latently infected individuals had detectable antibodies to a combination of the six potential protein candidates in a higher proportion of persons compared with TB patients. The presence of specific antibodies in sera from LTBI patients and their absence in sera from most TB patients suggest that the antigens used are preferentially expressed during the early phase of MTB infection, and it may be associated with inactive state. Positive strip test results found in some of the TB patients were expected as most patients go through a phase of latent infection before progression to active TB disease occurs. Antibodies to latency antigens induced during this latent phase may remain detectable thereafter. The sera of non-TB individuals were mostly negative. Thus, strip test based on the MTB latency proteins is a highly specific test for differentiating LTBI and TB samples as well as distinguishing LTBI and non-TB samples. Patients with no clinical manifestations should probably be diagnosed as having LTBI if the strip test result is positive. The false-negative strip test results could be understood in terms of the immunogenicity of the latency antigens used in the present study. They do not represent the whole spectrum of antigenicity of MTB. The possibility of generating false negativity by strip test could not be excluded. Clinical, bacteriological, and radiographic approaches and additional tests might be needed to draw a diagnosis conclusion.
The use of strip test for LTBI in this study is experimental. Testing was done retrospectively using stored frozen sera that passed through one freeze-thaw cycle. Additional research will be needed to determine whether strip test can be further improved and the value of test needs assessment in large studies recruiting fresh serum samples of patients.
Conclusion
Compared with the gold standard TST, strip test is more convenient and more specific. The strip test has low turnaround time and a relatively low rate of false positives that may be useful as a diagnostic test to rule-in clinical suspicion of LTBI. It can help overcome barriers to accessing LTBI diagnosis that could be reached before the patient progresses to overt disease and will avoid the consequences of unnecessary treatment giving high false-positives of TST. Use of strip test as a supplementary diagnostic aid in TST-positive cases helps support a diagnosis of LTBI among patients predominantly vaccinated with BCG in highly burdened TB and resource-constrained settings. However, this antibody-based method is still experimental for the detection of LTBI.
Sensitivity = 75.0% (24/32) Specificity = 88.1% (52/59) Positive predictive value = 77.4% (24/31) Negative predictive value = 86.7% (52/60)
[1]. Uplekar M, Weil D, Lonnroth K, Jaramillo E, Lienhardt C, Diaz HN, WHO’s new End TB StrategyLancet 2015 385:1799-801. [Google Scholar]
[2]. World Health Organization. Global Tuberculosis Report 2014 2014 Accessed on 25 February 2016Geneva, SwitzerlandWHOAvailable from: http://apps.who.int/iris/bitstream/10665/137094/1/97892415 64809_eng.pdf [Google Scholar]
[3]. Mack U, Migliori GB, Sester M, Rieder HL, Ehlers S, Goletti D, LTBI: latent tuberculosis infection or lasting immune responses to M. tuberculosis? A TBNET consensus statementEur Respir J 2009 33:956-73. [Google Scholar]
[4]. Tissot F, Zanetti G, Francioli P, Zellweger JP, Zysset F, Influence of bacilli Calmette-Guerin vaccination on size of tuberculin skin test reaction: to what size?Clin Infect Dis 2005 40:211-17. [Google Scholar]
[5]. World Health OrganizationUse of tuberculosis interferon-gamma release assays (IGRAs) in low- and middle-income countries 2011 Accessed on 25 February 2016Geneva, SwitzerlandWHOAvailable from: http://www.who.int/tb/features_archive/policy_statement_igra_oct2011.pdf [Google Scholar]
[6]. World Health OrganizationCommercial serodiagnostic tests for diagnosis of tuberculosis 2011 Accessed on 25 February 2016Geneva, SwitzerlandWHOAvailable from: http://whqlibdoc.who.int/publications/2011/9789241502054_eng.pdf [Google Scholar]
[7]. Kasempimolporn S, Thaveekarn W, Kerdpanich P, Skulpichetrat U, Saekhow O, Boonchang S, Performance of a rapid strip test for the serologic diagnosis of latent tuberculosis in childrenJ Clin Diagn Res 2015 9:DC11-DC14. [Google Scholar]
[8]. Leistikow RL, Morton RA, Bartek IL, Frimpong I, Wagner K, Voskuil MI, The Mycobacterium tuberculosis DosR reguron assists in metabolic homeostasis andenables rapid recovery from non respiring dormancyJ Bacteriol 2010 192:1662-70. [Google Scholar]
[9]. Roupie V, Romano M, Zhang L, Korf H, Lin MY, Franken KL, Immunogenicity of eight dormancy regulon-encoded proteins of Mycobacterium tuberculosis in DNA-vaccinated and tuberculosis-infected miceInfect Immun 2007 75:941-49. [Google Scholar]
[10]. Lin MY, Geluk A, Smith SG, Stewart AL, Friggen AH, Franken KLMC, Lack of immune responses to Mycobacterium tuberculosis DosR regulon proteins following Mycobacterium bovis BCG vaccinationInfect Immun 2007 75:3523-30. [Google Scholar]
[11]. Chen T, He L, Deng W, Xie J, The Mycobacterium DosR regulon structure and diversity revealed by comparative genomic analysisJ Cell Biochem 2012 114:1-6. [Google Scholar]
[12]. Prabhavathi M, Pathakumari B, Raja A, IFN-γ/TNFαratio in response to immune proteomically identified human T-cell antigens of Mycobacterium tuberculosis-The most suitable surrogate biomarker for latent TB infectionJ Inf 2015 71:238-49. [Google Scholar]
[13]. Sambrook J, Fritsch EF, Maniatis T, Molecular Cloning: A Laboratory Manual 1989 second editionNew YorkCold Spring Harbor Laboratory Press:1.53-1.87. [Google Scholar]
[14]. Pineiro R, Cilleruelo MJ, Garcia-Hortelano M, Garcia-Ascaso M, Medina-Claros A, Mellado MJ, Effect of nutritional status on tuberculin skin testingIndian J Pediatr 2013 80:271-75. [Google Scholar]
[15]. Nahid P, Pai M, Hopewell PC, Advances in the diagnosis and treatment of tuberculosisProc Am Thorac Soc 2006 3:103-10. [Google Scholar]
[16]. Laal S, Samanich KM, Sonnenberg MG, Zolla-Pazner S, Phadtare JM, Belisle JT, Human humoral responses to antigens of Mycobacterium tuberculosis: immunodominance of high-molecular-mass antigensClin Diagn Lab Immunol 1997 4:49-56. [Google Scholar]
[17]. Mustafa AS, Development of new vaccines and diagnostic reagents against tuberculosisMol Immunol 2002 39:113-19. [Google Scholar]
[18]. Sartain MJ, Slayden RA, Singh KK, Laal S, Belisle JT, Disease state differentiation and identification of tuberculosis biomarkers via native antigen array profilingMol Cell Proteomics 2006 5:2102-13. [Google Scholar]
[19]. Davidow A, Kanaujia GV, Shi L, Kaviar J, Guo X, Sung N, Antibody profiles characteristic of Mycobacterium tuberculosis infection stateInfect Immun 2005 73:6846-51. [Google Scholar]
[20]. Raja A, Uma Devi KR, Ramalingam B, Brennan PJ, Immunoglobulin G, A, and M responses in serum and circulating immune complexes elicited by the 16-kilodalton antigen of Mycobacterium tuberculosisClin Diagn Lab Immunol 2002 9:308-12. [Google Scholar]
[21]. Bothamley GH, Epitope-specific antibody levels demonstrate recognition of new epitopes and changes in titer but not affinity during treatment of tuberculosisClin Diagn Lab Immunol 2004 11:942-51. [Google Scholar]
[22]. Demissie A, Leyten EM, Abebe M, Wassie L, Aseffa A, Abate G, Recognition of stage-specific mycobacterial antigens differentiates between acute and latent infections with Mycobacterium tuberculosisClin Vaccine Immunol 2006 13:179-86. [Google Scholar]
[23]. Leyten EMS, Lin MY, Franken KLMC, Friggen AH, Prins C, Van Meijgaarden KE, Human T-cell responses to 25 novel antigens encoded by genes of the dormancy regulon of Mycobacterium tuberculosisMicrobes Infect 2006 8:2052-60. [Google Scholar]
[24]. Black GF, Thiel BA, Ota MO, Parida SK, Adegbola R, Boom WH, Immunogenicity of novel DosR regulon-encoded candidate antigens of Mycobacterium tuberculosis in three high-burden populations in AfricaClin Vaccine Immunol 2009 16:1203-12. [Google Scholar]
[25]. Hozumi H, Tsujimura K, Yamamura Y, Seto S, Uchijima M, Nagata T, Immunogenicity of dormancy-related antigens in individuals infected with Mycobacterium tuberculosis in JapanInt J Tuberc Lung Dis 2013 17:818-24. [Google Scholar]
[26]. Singh S, Saraav I, Sharma S, Immunogenic potential of latency associated antigens against Mycobacterium tuberculosisVaccine 2014 32:712-16. [Google Scholar]