Breast cancer is the second most frequent cancer in women after non-melanoma skin cancers. It is a public health problem for which the main prevention mechanisms include health awareness, early detection and effective treatment. Secondary prevention is accomplished by self-examination, clinical examination and mammography; the latter is the primary screening method that leads to reduced mortality [1,2]. There is no evidence that self-examination or clinical examinations reduce breast cancer mortality [3,4]. Moreover, diagnosis is still often delayed in developing countries [5].
Recent studies have questioned the impact of mammographic screening and its actual benefit. In 2014, the Canadian National Breast Screening Study published the results of 25 years of follow-up of patients who did or did not undergo mammographic screening; the study found no difference in overall survival between two groups. However, survival was higher in patients with non-palpable lesions [6].
Clinical examination is a simple, inexpensive, and non-invasive method for detecting breast cancer. While screening solely based on this method does not rule out the disease or reduce breast cancer mortality, the high specificity of an abnormal physical examination increases the likelihood of diagnosing a malignancy [7,8]. Moreover, the combination of clinical examination with mammography increases screening sensitivity.
Self-examination is not recommended owing to increased breast-related complaints as well as a greater number of biopsies for benign lesions [9-11]; however, it may play a role in increasing the awareness of women regarding the importance of breast cancer prevention, i.e., breast cancer awareness. The patient is encouraged to perform self-examination when convenient in order to perceive minimum changes in the breast and to actively seek medical care if any suspicious changes are detected [12].
Breast examination gloves were developed to facilitate the gliding of the examiner’s fingers on the breast in order to increase the sensitivity of both clinical and self-examinations. Many gloves are available in the market, such as Donna Glove®, Be-Glove®, Sensifemme®, Sensa Touch®, and others. Such gloves consist of three layers of 0.5-mm-thick polyurethane. The outermost layer is in direct contact with the breast, and mineral oil is contained between this layer and the middle layer. All components of the device conform to European Community Directives and Food and Drug Administration requirements (21 CFR 172.878 and 21 CFR 178.3620A) [13].
A cross-sectional study with 130 patients at the University of Rome Tor Vergata and the Central Hospital of Viterb was performed to evaluate the detection of palpable lesions by self-examination with and without the use of the gloves. Nodule detection was 100% in the group that used the glove compared to 48% in the group that did not. Patients found the product easy to use and noted that its availability increased awareness about breast cancer prevention [13].
Conversely, there have been published warnings against the use of such gloves as a replacement for mammography screening, stating that their effectiveness is inferior to that of mammography and emphasizing evidence that these devices are of very limited use for the detection of breast tumours [14].
Although there are some reports of increased touch sensitivity and greater detection of breast nodules while performing clinical breast examinations using gloves, there are no scientific studies investigating the benefits of these devices. Therefore, we performed the present study to address this issue.
Materials and Methods
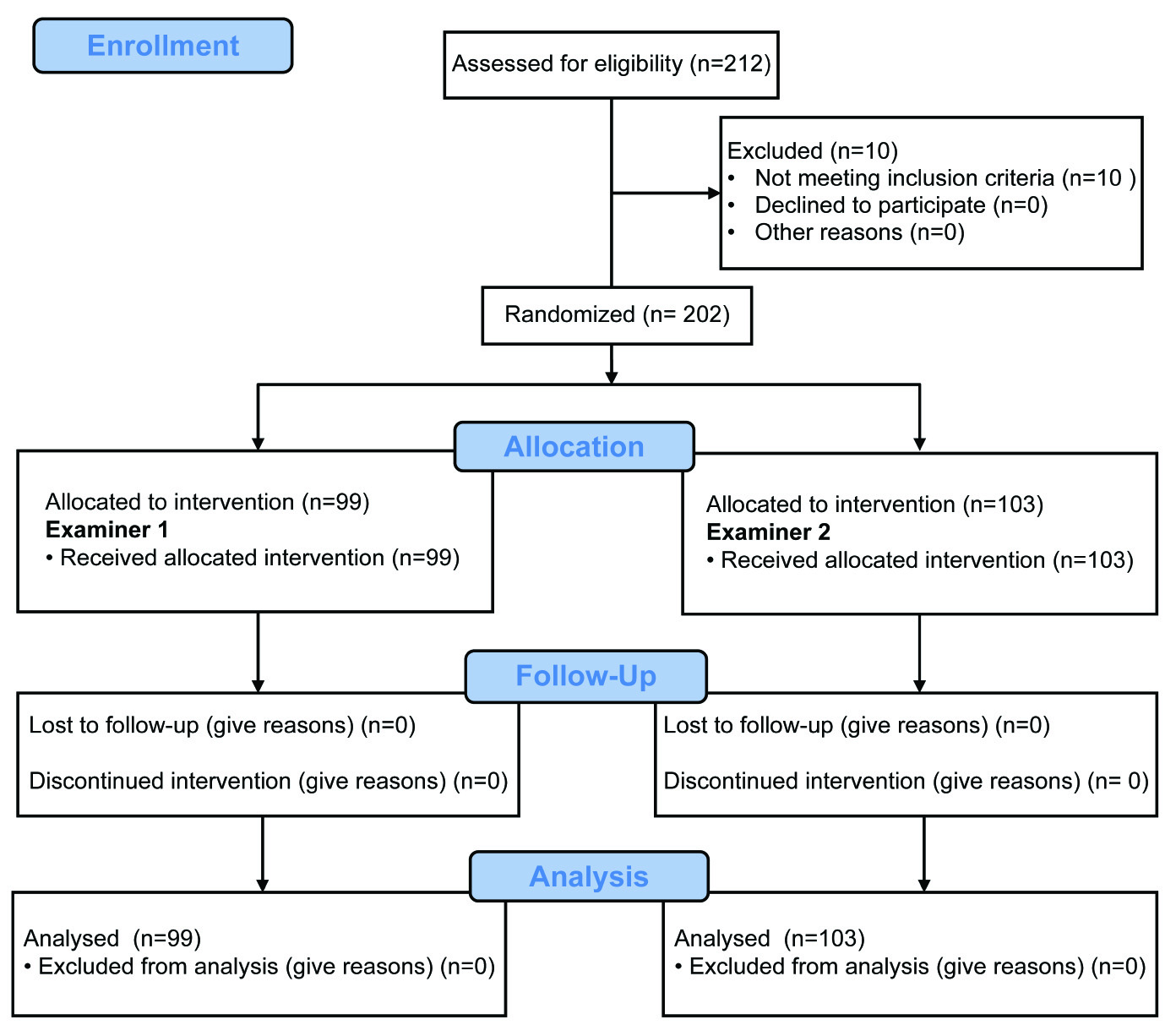
In this prospective study, patients from the Division of Mastology at the Department of Gynaecology, Federal University of São Paulo (UNIFESP) School of Medicine, Brazil, were randomly subjected to clinical breast examinations without (test 1) or with the Sensifemme® glove (test 2) by two different examiners (examiners 1 and 2). All patients underwent subsequent bilateral breast ultrasonography (test 3) to confirm or rule out the physical examination findings; this method is gold standard for diagnosis [Table/Fig-1]. The examiners were blinded to the results of the imaging tests. Ultrasonography was performed by a single observer (examiner 3) to decrease the false-positive rate. The Sensifemme® glove was developed by the Biotechnology Department of University of Alicante, Spain [Table/Fig-2]. The sample size was calculated using a type I error of 0.05, power of 0.9 and proportion of nodules diagnosed with glove unknown.
Study design to evaluate the clinical efficacy of using Sensifemme® glove in the diagnosis of breast nodules. Patients with and without nodules underwent tests 1, 2, and 3 by two different examiners (1 and 2).
The Sensifemme® glove used in the study. The device is made from the same material as other gloves and marketed for improving the detection of breast lumps.

Participants
Female patients aged 16 years and older were included, while patients with current or previous diagnoses of breast cancer were excluded. All participants signed an informed consent form, and the Ethics Committee of the UNIFESP approved the study. All the procedures followed were in accordance with the ethical standards of the responsible committee on human experimentation (institutional or regional) and with the Helsinki Declaration of 1975 that was revised in 2000.
Study End-points
The primary end-point was the success rate of diagnosing breast nodules using the glove. The secondary end-points were the sensitivity, specificity, accuracy and the positive and negative predictive values of the glove, as well as the concordance of conventional clinical examination with that using either the glove or ultrasonography. Other secondary end-points included the calculated learning curve for using the glove and the inter-observer variability when using the device.
Statistical Analysis
The following parameters were evaluated for assessing the validity of tests 1 and 2: sensitivity, specificity, accuracy, positive predictive value, and negative predictive value. The Chi-square (χ2) test was used to compare the observed and expected values and to assess whether the differences between values were likely to be actual or coincidental. The results of tests 1, 2, and 3 were compared with respect to the presence or absence of nodules, and the p-value <0.05 was considered statistically significant.
Analysis of the reproducibility of the diagnostic test was performed using the kappa concordance index. The ranges of the kappa values and the degree of concordance between each range used were as follows: less than 0.00: no concordance; 0.00–0.20: poor concordance; 0.21–0.40: fair concordance; 0.41–0.60: moderate concordance; 0.61–0.80: good concordance; 0.81–0.99: excellent concordance; and 1.00: perfect concordance [15]. All data were tabulated in a Windows Excel spreadsheet (Microsoft Excel 2011) and statistically analysed using Minitab software version 17.3.
Results
The study evaluated 202 patients with a mean age of 43.7 years; 152 patients were found to have breast nodules and 50 patients did not. Moreover, 298 breast lesions were diagnosed after clinical examination and imaging, representing 1.96 nodules per subject.
The mean size of the nodules was 1.5 cm and the mean distance of the nodule from the skin was 0.68 cm; both were measured by ultrasonography. The diagnosed lesions were predominantly nodular; 80% were solid nodules while 20% were cysts. Examiners 1 and 2 inspected a similar number of patients (99 and 103, respectively). The patients’ characteristics were homogeneous with respect to age, breast size, and the number of nodules present.
Diagnostic Test Results
In total, 161 nodules were detected by conventional clinical examination, while 203 were detected using the glove. This indicated a 14% increase in the rate of diagnosis of nodules when using the glove, given that the total number of nodules found by ultrasonography was 298 [Table/Fig-3]. The sensitivity, specificity and accuracy for subjects examined conventionally or using the glove are shown in [Table/Fig-4]. Comparing the detection of breast nodules when using tests 1, 2 and 3 showed that using the glove increased the rate of diagnosis; however, the results were inferior to those achieved with ultrasonography.
Diagnoses of True-Positive (TP) and True-Negative (TN) findings breast nodules with clinical examination, clinical examination with the Sensifemme® glove, and breast ultrasonography.
| Variables | Clinical examination TEST 1 | Clinical examination with the Sensifemme® glove TEST 2 | Ultrasound test 3 |
|---|
| Nodule present (TP) | 161 | 203 | 298 |
| Nodule absent (TN) | 39 | 29 | 50 |
Results of the diagnostic tests with clinical examination (test 1) and clinical examination with the Sensifemme® glove (test 2).
| Results of diagnostic tests | clinical examination (TEST 1) | clinical examination with the Sensifemme® glove (TEST 2) |
|---|
| Sensitivity | 54% | 68% |
| Specificity | 78% | 58% |
| Accuracy | 57% | 66% |
| Positive predictive value | 93% | 90% |
| Negative predictive value | 22% | 23% |
| False-positive | 22% | 42% |
| False-negative | 46% | 31% |
| True-positive | 54% | 68% |
| True-negative | 78% | 58% |
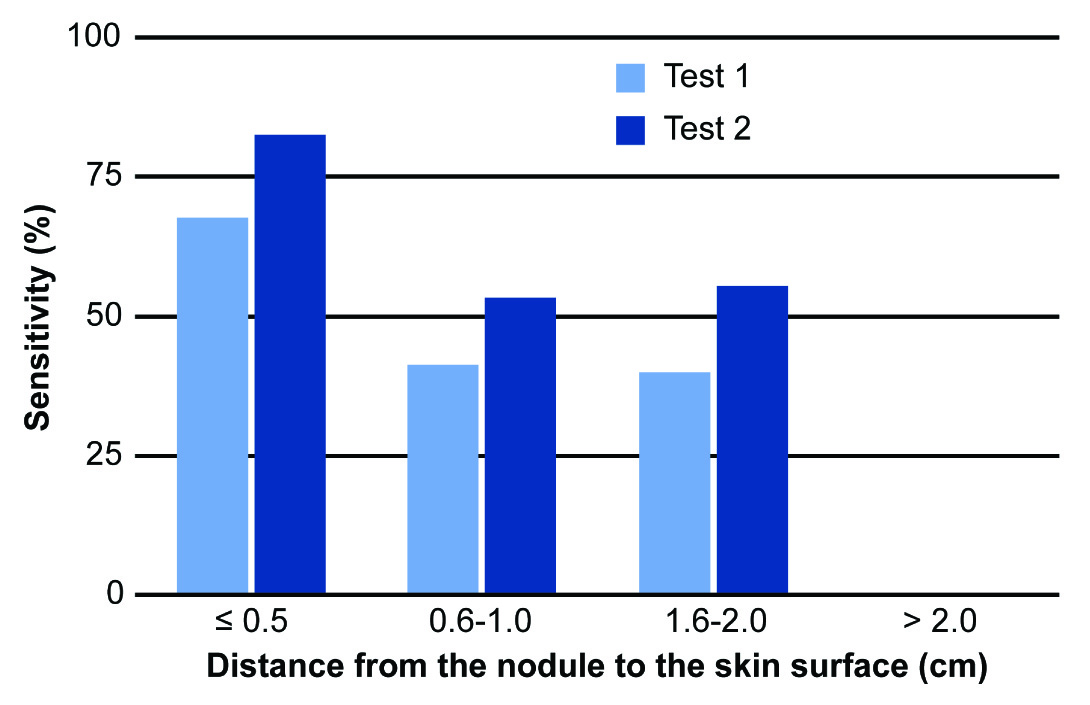
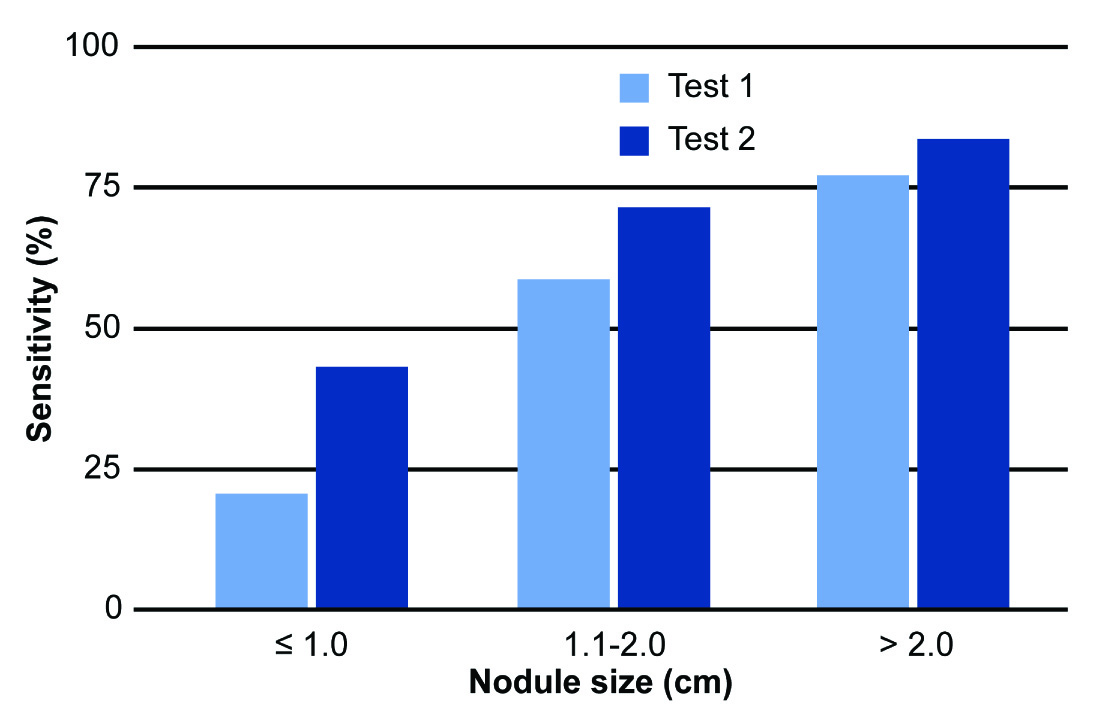
Statistical analysis revealed significant differences between tests 1 and 3 (χ2= 370.795; p<0.001), between tests 2 and 3 (χ2= 127.896; p<0.001) and between tests 1 and 2 (χ2 = 33.877; p<0.001). These results show that using gloves results in superior efficacy in diagnosing breast nodules compared to conventional clinical examination. The sensitivities of clinical examinations with and without gloves decreased significantly as the distance between the nodule and the skin increased. This shows the difficulty of detecting small, deep changes in fibroglandular tissues during physical breast examination. However, the sensitivity of the examination when using the glove was far superior to conventional clinical examination, especially for nodules up to 0.5 cm deep (83% vs. 68%, respectively) [Table/Fig-5]. The size of the nodule was also a variable that directly influenced the sensitivity of diagnostic tests 1 and 2; the largest differences occurred with nodules ≤1.0 cm, with sensitivities of 21% and 44%, respectively [Table/Fig-6].
Comparison of the sensitivity of the diagnosis of breast nodules in tests 1 (conventional breast examination) and 2 (examination using the Sensifemme® glove) in relation to the distance from the nodule to the skin surface, as measured by ultrasonography.
Comparison of the sensitivity of the diagnosis of breast nodules in tests 1 (conventional breast examination) and 2 (examination using the Sensifemme® glove) in relation to nodule size.
Concordance between Diagnostic Tests
The concordance between tests 1 and 2 was moderate, with a kappa index of 0.58. However, the analysis of concordance between clinical examination and ultrasonography (test 1 vs. test 3) and between examination with the glove and ultrasonography (test 2 vs. test 3) produced kappa indices of 0.15 and 0.16, respectively, indicating poor concordance.
Learning Curve for Performing Clinical Breast Examinations with the Glove
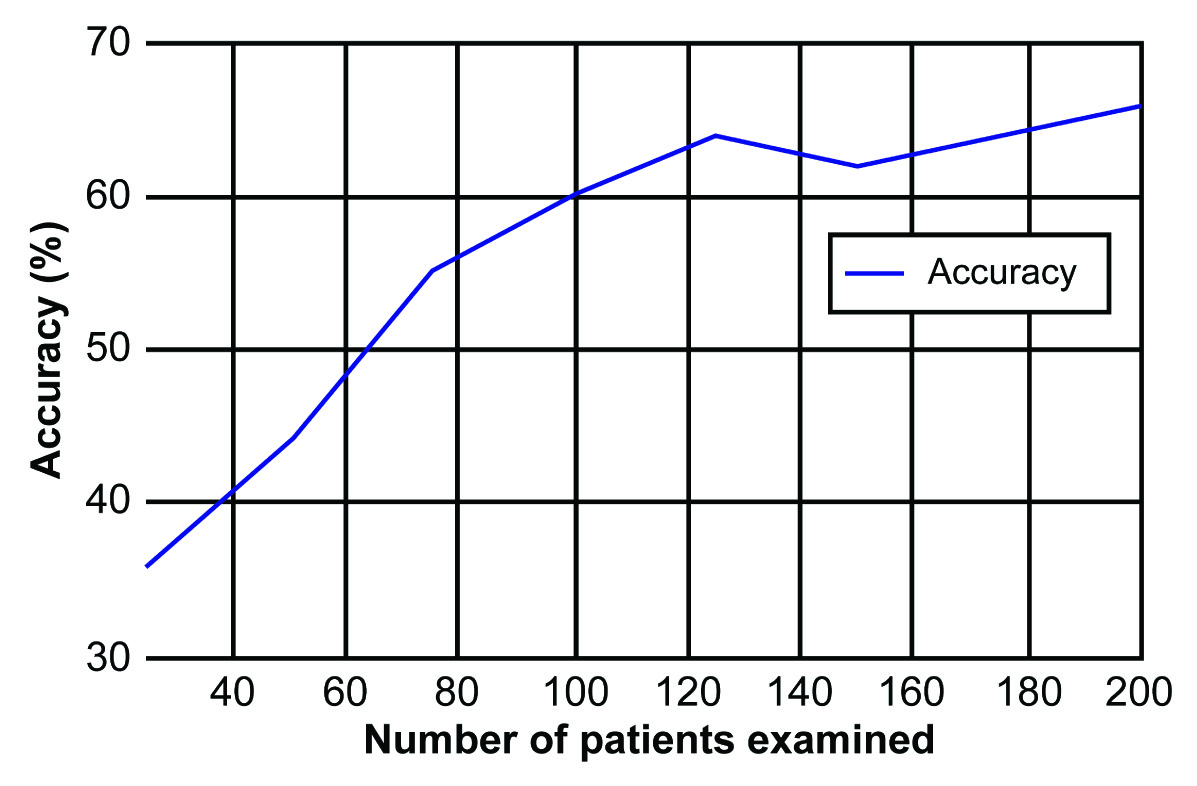
The examiners’ learning curve for using the glove was calculated considering the accuracy of nodule detection in relation to the number of patients examined. The accuracy of physical examination with the glove was much lower in the first 25 patients examined (36%) but increased throughout the duration of the study. When analysing for the accuracy of nodule diagnosis with the glove, a rising curve was observed representing the first 100 women, after which the accuracy plateaued [Table/Fig-7]. Compared with the overall accuracy of conventional clinical examination (57%), the accuracy when using the glove was superior to that when performing conventional clinical examination after examining only 100 patients.
The learning curve for clinical examination using the Sensifemme® glove: accuracy in relation to the number of patients examined.
Inter-observer Variability in the Clinical Efficacy of Breast Examinations with the Glove
The accuracies of examinations with the gloves by examiners 1 and 2 were 64% and 69%, respectively, with no discordance between them; both examiners showed superior clinical examination accuracy when using gloves compared to conventional examination (57%). The kappa indices of examiners 1 and 2 when comparing to ultrasonography were 0.12 and 0.20, respectively. Hence, the diagnostic tests correlated poorly with ultrasonography regardless of the examiner.
Discussion
The greater sensitivity of clinical breast examination with the glove (68% for test 2 vs. 54% for test 1) is likely the result of the easier gliding of the glove along the breast tissue, facilitating the detection of nodules during the physician’s examination. However, this was at the expense of an increased rate of false-positives (42% using test 2 compared to 22% using test 1), which can lead to prescribing complementary tests, short clinical follow up periods, unnecessary biopsies, increased costs, and patient anxiety.
The specificity of the examination with test 2 was lower compared to the control group (58% vs. 78%, respectively). The accuracy when detecting breast nodules during examination with the glove was 66%, which was significantly higher than conventional clinical examination (57%). This result illustrates a greater efficacy when using the glove. The predictive values of the study do not apply universally because they are directly influenced by the prevalence of the disease in any particular study population.
Using the glove increased the sensitivity and accuracy of detecting breast nodules by 14% and 9%, respectively, albeit with a higher rate of false-positive findings. Among the variables analysed, the distance from the nodule to the skin and the size of the lesion most influenced the sensitivity of the test. The sensitivity of the clinical examination with or without the glove decreased substantially for deeper lesions and increased in direct proportion to the nodule size. This is consistent with data from the literature [7,12,16]; however, the consistency variable was not assessed in the present study.
The high sensitivities identified in our study (54% for test 1 and 68.1% for test 2) may be due to sampling bias. Our cohort was not representative of the general population, as advanced cases and patients who experienced abnormal examination results may have been included among subjects who were referred to our study; this may have resulted in the overestimation of the sensitivity of the test compared to if it had been performed in the general population [17].
Ultrasonography is the examination of choice for palpable lesions, breasts of young patients, and dense breasts; it is also used to differentiate between solid and cystic lesions and to guide invasive procedures [18,19]. However, this technique is operator-dependent and is associated with false-positive lesions [20,21]. Moreover, the mean age of the women in the present study was 43.7 years, an age group for which the standard screening technique is mammography [22], which was not evaluated.
The use of the gloves requires a learning curve; examination of a 100 women was necessary for sufficient training to improve the detection accuracy to a level that justifies their use compared to conventional examination. The gloves are sold with the goal of increasing the sensitivity of breast self-examination, which could result in the early diagnosis of breast cancer. However, studies have shown that self-examination is not recommended for breast cancer screening [1], as detected lesions are usually symptomatic by the time they are felt and are associated with decreased odds of survival [6,23]. Conversely, early diagnosis of cancer is directly associated with the detection of subclinical lesions by mammography [6].
Breast cancer awareness is a recommended practice for health education and familiarity with one’s own body. Awareness of breast diseases can sensitise patients to any changes in their breasts and direct them to seek medical care promptly [24]. Annual clinical examinations are indicated for breast cancer screening, and usually involved bilateral mammography [24,25]. To that end, the glove can make a positive impact, as it can assist in improving the accuracy of clinical examinations without diminishing the importance of mammography [25].
Conclusion
Using the glove during clinical examination of the breast for lumps is feasible, beneficial and inexpensive; however, it is not a substitute for traditional breast cancer screening. The glove can be used as an additional tool to improve the efficacy of clinical examination, and to alert the individual to seek medical advice when any change is detected.
Using the glove to perform clinical breast examinations was more effective than conventional examination for the diagnosis of breast nodules, and exhibited increased sensitivity and accuracy. However, this was at the expense of a higher false-positive rate, which can lead to additional tests, unnecessary biopsies, and patient anxiety. The concordance of the clinical examinations (with or without the glove) with ultrasonography is weak independent of the examiner. Moreover, there is a steep learning curve when using the glove.