FD is one of the most common causes of gastrointestinal discomfort. It is defined by Rome III criteria as syndrome with one or more of following symptoms which include bothersome postprandial fullness, early satiation, epigastric pain and burning with no evidence of structural disease as seen in upper endoscopy that is likely to explain the symptoms. These criteria should be fulfilled for at least three months with symptom onset, at least six months previously [1].
Aetiology of FD is poorly understood. Local factors like delayed gastric emptying, impaired proximal gastric accommodation to food, gastric hypersensitivity with decreased pain threshold during stomach distension are blamed for the same [2,3]. Activation of immune system with release of mediators like cytokines, nitric oxide, histamine and protease which interfere with function of enteric nerves is the another proposal [4]. According to some studies H Pylori infection can be responsible for FD not only by virtue of bringing out gastric but duodenal inflammation as well [5]. Much is being said about duodenal epithelial function, mucosal defense barrier and appropriate signaling systems in patients with FD [6]. Potential role of duodenal IEL and eosinophils in lamina propria has also been described in relation to FD [7,8].
In view of this, we thought that study of histomorphological spectrum of duodenal mucosal pathology with special reference to IEL and eosinophils in lamina propria will be worthwhile in evaluating cases of FD.
Materials and Methods
Study design: This prospective study was performed at a Tertiary Care Hospital (BJGMC and SGH Pune, Maharashtra, India) for a period of two years from January 2011 to December 2012. This case control study comprised of 50 patients of FD defined by Rome III criteria and 30 age and sex matched controls. The controls comprised of patients in whom oesophago-gastro-duodenoscopy (OGDscopy) was done for indications other than FD. After seeking Ethics Committee approval and informed consent, both patients and controls were subjected to OGDscopy. Patients with history of peptic ulcer disease, gastrointestinal malignancy, previous gastric surgery, drug intake and upper gastrointestinal bleeding were excluded from the study.
The study group was evaluated for socio demographic variables (e.g., age, sex), dietary habits, drug history (e.g., NSAIDs, antacids) and gastrointestinal symptomatology. OGDscopy was done and findings were noted. Two biopsies were taken, one each from gastric antrum and second part of duodenum. Rapid urease test was performed for H. pylori status on gastric biopsies in gastroscopy room using CLO test kit. A positive result was indicated by colour change from yellow to pink. Duodenal biopsies were fixed in 10% formalin and processed routinely. The sections were cut at 5 micron using rotary microtome and stained with H&E stain, Geimsa and Methylene blue stain.
Detailed histopathological examination of duodenal biopsies was done for grading of inflammation and quantification of duodenal IEL and eosinophils in lamina propria as described below.
Grade of inflammation: Duodenal inflammation was graded by two methods. The subjective grading (as mild, moderate and severe inflammation) was based on overall impression of density of infiltrate in lamina propria and villous morphology. Second is objective grading.
While grading inflammation objectively we used following criteria: density of infiltrate in lamina propria when compared with that in control, counts of IEL and eosinophils in lamina propria and villous architecture [Table/Fig-1]. The findings of metaplasia and presence of H. pylori in duodenum were noted separately. Scoring system for objective grading of duodenal inflammation has been given in [Table/Fig-1].
Scoring system for objective grading of duodenal inflammation.
| Infiltrate in lamina propria | IEL count / 100 Entero- cytes | Eosinophil count/ 5 HPF | Villous architecture | Score for each parameter |
|---|
| Within normal limits | Up to 18 | Up to15 | Normal | 0 |
| Overall increased density of cells | 19-30 | 16-30 | Focal blunting | 1 |
| Overall increased density of cells + presence of polymorphs | 31- 40 | 31-40 | Diffuse blunting | 2 |
| Overall increased density of cells + Lymphoid follicle or dense focal collection | >41 | 41-50 | Focal/Diffuse blunting | 3 |
| Overall increased density of cells + presence of polymorphs +Lymphoid follicle or dense focal collection of lymphocyte | >50 | >50 | Focal/Diffuse blunting | 4 each |
On summation of individual scores, score of 1 to 4 was graded as mild inflammation, 5 to 8 as moderate inflammation and score more than (>) 8 as severe inflammation.
IEL were counted per 100 enterocytes lining the villous or by villous tip method. Eosinophils were counted in five consecutive non overlapping High Power Fields (HPF) in lamina propria.
Ten cases with IEL count higher than 27/100 enterocytes were further evaluated with IHC for CD4 and CD8 typing of lymphocytes.
Statistical Analysis
The data collected was analysed using SPSS-17.0 software. Chi-Square test was applied to find out significance of difference between means. Correlation t-test and logistic regression analysis was used to find out the correlation between different parameters. The p-value of < 0.05 was considered to be significant.
Results
Age and Sex distribution: The mean age for control was 36.3 years with age range of 20-51. The mean age for FD cases was 34.2 years with age range of 20-63 years. There was slight male preponderance in group of FD with 32 male patients and 18 female patients.
Gastric H. pylori status based on rapid urease test: Rapid urease test was positive in 24 (48%) cases of FD. Staining of H pylori was better with methylene blue stain than with Giemsa stain.
Duodenal Inflammation: When biopsies were examined subjectively in patients of FD, it was possible to rate them as mild inflammation in 23 cases (46%), moderate inflammation in 24 cases (48%) and severe in three cases (6%). Grading of inflammation changed as follows when we resorted to objective grading system. It was mild grade for 31 cases (62%) and moderate grade for 19 (38%) cases in FD.
Villous atrophy: Variable villous abnormality, the form of focal changes of mild flattening of villi was noted in eight cases. Of these, seven were associated with gastric H. pylori infection. The range of IEL was from 12 to 37/100 enterocytes with mean of 25.62. The same for eosinophil count/5 high power field was 12 to 39 with mean of 24.5. One case each with gastric H. pylori positivity and negativity had normal values for IEL. One gastric H. pylori positive case showed normal eosinophilic count in duodenum. Two casesn with gastric H. pylori infection were associated with moderate degree inflammation. One of these had goblet cell metaplasia and presence of lymphoid follicles in mucosa in addition and highest values of eosinophils in the group. The other case had highest value of IEL in the group. Closer look at the observation of cases of villous atrophy suggest that, two cases of variable villous abnormality were seen with food allergy with or without gastric H. pylori infection while rest six cases were associated with H. pylori infection.
Lymphoid aggregates: It was noted in two cases and both these cases had variable villous abnormality. The lymphoid follicles were seen in areas away from variable villous abnormality (focal villous atrophy). Both these cases were positive for antral H. Pylori. One case had mild inflammation while the other had moderate inflammation.
Metaplasia: Colonic type of goblet cell metaplasia was noted in two cases. Both showed antral H. pylori positivity and moderate degree inflammation. In one case it was associated with variable villous abnormality and lymphoid follicles. The counts for IEL in these cases were 30 each and that for eosinophils 39 and 88 each.
H. Pylori in duodenum: Presence of few H. pylori organisms was noted in two cases.
Intraepithelial Lymphocytes: The mean IEL counts for control was 9.3± 2.5 with range of 5-18 / 100 enterocytes, while that of FD cases was 26.18±5.9 with range of 11-37 / 100 enterocytes. About 70% cases (n=35) showed IEL count to be in the range of 21-30 lymphocytes / 100 enterocytes [Table/Fig-2].
Overall status of H. Pylori, IEL and eosinophils.
| Gastric H. Pylori status | Status of IEL and Eosinophils | Inference |
|---|
| Rapid Urease test positive n= 24 | Raised IEL and Eosinophil CountRaised IEL and normal Eosinophil count | 36% (n=18)4% (n= 02) | H. pylori infection |
| Normal IEL and raised Eosinophil CountNormal IEL and Eosinophil count | 6% (n= 03)2% (n= 01) | H. pylori infection /???Food Allergy ?Motor dysfunction |
| Rapid Urease test Negative n = 26 | Raised IEL and Eosinophil Count | 32% (n=16) | Other infections / Food Allergy |
| Normal IEL and raised Eosinophil Count | 18% (n= 09) | Food Allergy |
| Normal IEL and Eosinophil Count | 2% (n= 01) | ?Motor dysfunction |
IEL count was increased in 36 (72%) cases of FD when compared to higher limit of our own controls. Of these 20 cases (55.55%) were rapid urease positive. The difference between mean IEL count of H. pylori positive cases and controls was statistically significant with p-value <0.001, while that between H. pylori negative cases and controls was not statistically significant.
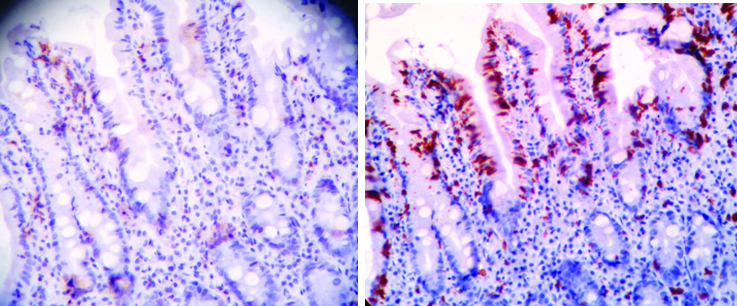
On immunohistochemical evaluation of ten antral rapid urease positive cases with increased IEL counts (> 27/100 enterocytes), all showed increased CD8+lymphocytes compared to CD4+ lymphocytes both in intraepithelial location and in lamina propria [Table/Fig-3].
Comparison of CD4 (a); and CD8 (b) lymphocytes (IHC 10X).
Eosinophil count: Mean eosinophil count was 9.5±2.04 for controls with range of 6-15/ HPF and that of patients with FD was 40.7±26.9 with range of 6 to 134/ 5 HPF. In FD, maximum observations (n=19) were in the range of 21 to 30 eosinophils/5 HPF [Table/Fig-4].
Intraepithelial lymphocytes (H&E, 40X).
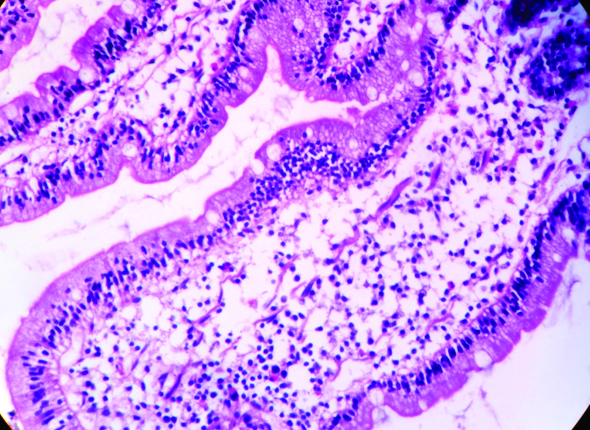
Eosinophils in lamina propria (H&E, 40X). (Images left to right)
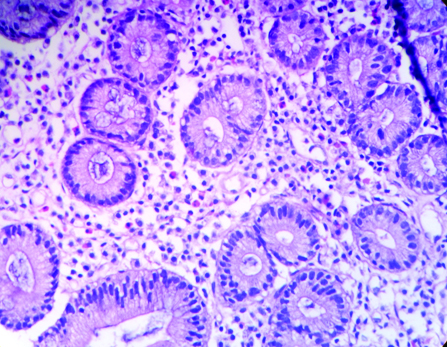
When compared with highest values of our own controls, 92% (n=46) patients with FD showed duodenal eosinophilia. Eosinophilia was seen in 21 gastric rapid urease positive cases and 25 out of 26 rapid urease test negative cases. The difference between mean eosinophilic count of H. pylori positive cases and controls was statistically significant with p-value 0.012.
Correlation of variables of duodenal inflammation:
IEL correlated positively with presence of gastric H. pylori with Chi-square value of 11.45 and p-value of <0.001 with CI range of 2.2 to 28.7. This wide range of CI can be attributed to moderate sample size.
Duodenal eosinophil count showed positive correlation with positive gastric H. pylori status with Chi-square value of 6.2 and p-value of 0.012 with CI range of 1.59 to 3.04.
On attempting linear regression analysis of different morphological variables of duodenal biopsy with enter method, only IEL showed positive correlation with variables like grade of inflammation, eosinophil count in lamina propria, lymphoid aggregates, metaplasia and villous atrophy. The range of CI however was wide with values of 2.15 to 41.75 perhaps due to moderate sample size.
Discussion
FD is a heterogeneous disorder frequently seen in general population. It’s prevalence in India is 13.4% [9]. Present study was conducted to find out possible immune mechanism underlining FD. This was done by quantifying and characterizing duodenal intraepithelial lymphocytes both in H. pylori negative and positive patients. In our study, we also tried to find out role of eosinophils in FD.
Mild to moderate degree duodenal inflammation was an invariable feature noted in our patients of FD suggesting its role in symptomatology. Pathogenesis and significance of duodenitis in FD is being elucidated recently. It is suggested that gut-brain-microbial axis plays an important role in functional gut disorders [10]. Recent studies also show persistence of duodenal inflammation in post infection FD [11]. Traditionally stomach pathology is believed to be responsible for dyspeptic symptoms. Recent studies however suggest that the site of pathology may instead be duodenum. Abnormally raised intra epithelial lymphocytes and eosinophils are frequently noted in duodenum in H. pylori dyspepsia [12]. Suzuki H et al., have stated that H. pylori infection evokes inflammation not only in gastric mucosa and muscularis but also in duodenal mucosa [13].
According to some studies, the persistence of inflammation results from impaired ability of immune system to terminate the inflammation after acute infection by H. pylori, Salmonella or Giardia organisms. IEL by virtue of their strategic location play a great role in preservation of mucosal integrity and maintenance of tolerance to oral particulate or soluble antigens [14].
Hayat M et al., have noted raised IEL in second part of duodenum in 30% cases of H. pylori gastritis which persisted even after H. pylori were eradicated from gastric location [15].
Raised duodenal IEL [Table/Fig-2] was a feature noted in 72% of our cases of FD. In gastric H. pylori positive group, it was seen in 83.33% cases. Experience is shared by others. Lorenzo M et al., in addition demonstrated that majority of duodenal IEL to be CD8+, TIA-1+ and Granzyme B+ which is considered to be activated cytotoxic phenotype [7,16]. Incidentally the duodenal IEL in present study showed markedly raised expression of CD8+ when compared with CD4. This suggests activation of CD8+ lymphocytes without priming with CD4+ cells.
Triad of villous abnormality, raised IEL and chronic duodenal inflammation has been referred as “celiac” lesion in literature. Numerous aetiological agents can elicit such tissue response which mimics celiac disease [17-19]. Such lesions were seen in six of our H. pylori positive cases of FD. On clinical grounds we have ruled out possibility of latent celiac disease. These cases can be attributed to H. pylori infection.
Closer look at the observation of cases of villous atrophy suggest that, two cases of variable villous abnormality were seen with food allergy one of which had gastric H. pylori infection.
More than 70% of Indian population is chronically infected by H. pylori [20]. Prevalence of H. pylori in present series of FD was 48%. Same in a study from Kashmir has been shown to be 58% [21]. We found two cases of goblet cell metaplasia in duodenum in our gastric H. pylori positive patients of FD. Goblet cells can express both antigens of gastric and intestinal mucosa and are considered to represent local precursors of gastric metaplasia [20].
Normal value of IEL in duodenum is subject of individual variability. In present study, highest values of control were 18/100 epithelial cells. Hayat M et al., found it be less than 20/100 epithelial cells [15]. The count of 25 is considered as pathological, between 25 to 30 as border line and that of 30 and more as overtly pathological. Findings in present study are in accordance with this view. The IEL counts of 30 and above were seen in seven cases, between 25 to 30 in 17 cases, between 20 to 24 in 11 cases and a count of 19 in one case of FD.
Very interestingly a spectacular finding of almost invariable duodenal eosinophilia [Table/Fig-4] was noted in our patients of FD (92%). Eosinophils which have different effect or functions are very aptly considered biomarker of FD. They play a great role in allergic inflammation, in host defence mechanism against helminths and in dealing with bacterial and viral infections. H. pylori infection influences duodenal eosinophils. Raised duodenal eosinophils are noted in patients of FD and are suggested to be related to early satiety. It is now almost well established that eosinophils and mast cells are key components of gut hypersensitivity disorder or functional gastrointestinal disorders [22, 23]. FD and Irritable Bowel Syndrome (IBS) are functional disorders and have overlapping symptoms. IEL are of no help in discriminating them but duodenal eosinophils can do this job. While duodenal eosinophilia is linked with FD, mast cell hyperplasia is a feature of IBS [24]. Incidentally both these counts are increased in children with FD triggered by food allergen [25].
Walker MM et al., have suggested that eosinophil-mast cell-neural pathway is involved in functional gastrointestinal disorders [24]. It is a hypersensitivity reaction triggered by pathogen, food or other allergen in which eosinophils or mast cells can up regulate serotonin release which modulates enteric and central nervous system and release lipid mediators and leucotriens. These are very potent stimulators for smooth muscle contraction and thereby responsible for dyspeptic symptoms like abdominal pain and bloating.
Some authors have found a significant correlation between the appearances of symptoms of food hypersensitivity and the presence of IgE-bearing cells and activated eosinophils in gastrointestinal tract. These patients exhibited negative skin prick test and absence of IgE antibodies to the offending food. The gastrointestinal symptoms seen in these patients can be attributed to a localized IgE-mediated response [26].
Considering all these experiences, however simplistic it may feel we have attributed finding of raised duodenal IEL and or eosinophilia in gastric H. pylori positive patients to H. pylori infection, that with raised IEL in gastric H. pylori negative group to some other infection, that of normal IEL and raised eosinophil in gastric H. pylori negative patients to food allergy and that with normal IEL and eosinophil to pure motor dysfunction as shown in [Table/Fig-2]. Decreased clearance and increased exposure to duodenal lipids or acid due to reduced duodenal motor response and altered duodeno- jejunal motility are some of the motor dysfunction factors explaining FD symptoms [27,28]. We had two such cases which had normal IEL and eosinophil count. One of such cases was H. pylori positive and the other was H.pylori negative. The findings are summarized in [Table/Fig-5].
It will not be too pragmatic to state that screening and eradication of H. pylori along with anti allergic treatment should be a primary concern in treating patients with FD. A simple measure of small and frequent meals will also go long way in treating patients of FD.
Limitation
Moderate sample size was the main limitation of the study along with lack of definitive evidence to support the role of food allergy and motor dysfunction in aetiology of FD.
Conclusion
This comprehensive study of duodenal morphology provided some insight in pathogenesis of FD and role of H. pylori in it. It definitely makes sense in screening and eradicating H. pylori in patients with FD.