Introduction
Prostate cancer is the most commonly diagnosed non-cutaneous cancer [1] and its incidence is rising rapidly in most countries including India [2]. As prostate cancer mostly affects older people, its incidence is likely to increase in near future as average life expectancy is on a rise. Men over 50 years have about 40% chance of having cancer in the prostate, regardless of nationality, race or ethnicity [3]. The aetiology of prostate cancer is unclear, although current evidence suggests that it is the result of multiple factors such as ethnic, environmental, genetic, hormonal and diet [4]. A significant hereditary element in the susceptibility of development of prostate cancer has been recognized by various genetic studies. These studies have revealed two-fold to three-fold increased risk of developing prostate cancer in men who have a first-degree relative (father, brother, son) with prostate cancer as compared to men with no family history [5]. Numerous genes’ polymorphic sites have been studied to establish an association with prostate cancer to predict its risk of development which yielded inconsistent results. These genes include genes encoding the Androgen Receptor (AR), CYP17, and 5α-reductase type 2 (SRD5A2), CYP3A, Prostate Specific Antigen (PSA), Insulin-like Growth Factor (IGF)-1, and IGF-binding protein 3 [6,7]. But, prostate cancer lacks an established genetic marker for predicting susceptibility and progression.
Besides its role in maintaining calcium homeostasis, vitamin D is now known to affect cell growth and differentiation, immune function and can protect against cardiovascular disease, infections, cancer and autoimmune diseases such as multiple sclerosis [8-12]. By expressing 1α-hydroxylase, prostatic epithelial cells synthesize active form of vitamin D i.e., 1,25-dihydroxyvitamin D3 which plays a growth regulating role in prostate [13]. In addition, calcitriol exhibits anti-proliferative and pro-differentiating activities in malignant prostate cell lines and in some in vivo models of prostate cancer [14-17]. Mechanism of action of 1,25(OH)2D3 is mediated by its binding to VDR. VDR functions as a heterodimer, generally with the retinoid X receptor for regulation of vitamin D target genes. The gene encoding the VDR is located on chromosome 12q13.11, contains 14 exons and spans approximately 75 kilobases of genomic DNA [18-20]. Various polymorphisms have been identified in the VDR gene such as ApaI, BsmI, TaqI, FokI, Tru9I, cdx2 and EcoRV. FokI (rs 2228570) polymorphism, present in exon 2, produce a shorter VDR protein which is more effective in transactivation of the 1,25(OH)2D3 signal [20,21]. TaqI (exon 9) and ApaI (intron 9) polymorphisms, however, do not alter the amino acid of the VDR protein; but, they may influence gene transcription and mRNA stability [22].
There is paucity of knowledge about VDR gene polymorphism and its association with prostate cancer in Indian population. Therefore, we conducted this study to investigate possible association of three VDR gene polymorphisms (FokI, TaqI and ApaI) with prostate cancer.
Materials and Methods
This case-control study was conducted in the Department of Biochemistry, Vardhman Mahavir Medical College and Safdarjung Hospital, New Delhi, India, in association with the Department of Urology, at a tertiary care hospital. Clearance from Institutional Ethical Committee was obtained preceding the study. Cases included 120 newly diagnosed patients of prostate cancer which were histologically confirmed. All cases were in advance stage of the disease. Advanced prostate cancer was defined according to Surveillance Epidemiology and End Results (SEER) 1995 pathologic and clinical extent of disease codes 41–85 [23]. Control groups consisted of group I which included 120 normal age and sex matched healthy controls selected from the volunteers with Prostate Specific Antigen (PSA) levels <4.0ng/ml and no history of prostate cancer among their first degree relatives and group II consisted of 120 first degree relatives of prostate cancer patients. First degree relatives include prostate cancer patients’ sons or their brothers. All normal healthy controls as well as first degree relatives were screened for PSA level (normal <4.0 ng/ml). Selected controls did not have any history of cancer and/or prostate surgery. Written informed consent was taken from all subjects. Blood samples were collected in vials containing EDTA K2 anticoagulant and stored at -80°C till further analysis. Total genomic DNA was isolated from whole blood using the method described by Daly AK et al., [24]. The required region of VDR gene from the genomic DNA was amplified by PCR in MJ Research PTC-100™ (Peltier Thermal Cycler) using the primers as follows:
Primers for FokI: (Harris SS et al., [25])
Forward: 5’- AGC TGG CCC TGG CAC TGA CTC TGC TCT-3’
Reverse: 5’- ATG GAA ACA CCT TGC TTC TTC TCC CTC-3’
Primers for TaqI and ApaI: (Riggs BL et al., [26])
Forward: 5’- CAG AGC ATG GAC AGG GAG CAA-3
Reverse: 5-GCA ACT CCT CAT GGC TGA GGT CTC-3
For FokI, PCR product of 265 bp was obtained and verified using a 2% agarose gel. The PCR product was digested with Fok-I restriction enzyme obtained from New England Biolabs (NEB). The FF genotype lacked a Fok-I site and showed only one band of 265 bp. The ff genotype generated two fragments of 196 and 69 bp. The heterozygote displayed three fragments of 265, 196 and 69 bp, designated as Ff.
[Table/Fig-1] shows Agarose gel picture of electrophoresis pattern of restricted enzyme digested PCR products showing Heterozygous (Tt) in lane 1 and 3, Homozygous (TT) in lane 2, Heterozygous (Aa) in lane 4, Homozygous (AA) in lane 5 and 100 bp marker in lane 6.
Agarose gel picture of electrophoresis pattern of restricted enzyme digested PCR products showing Heterozygous (Tt) in lane 1 and 3, Homozygous (TT) in lane 2, Heterozygous (Aa) in lane 4, Homozygous (AA) in lane 5 and 100 bp marker in lane 6.
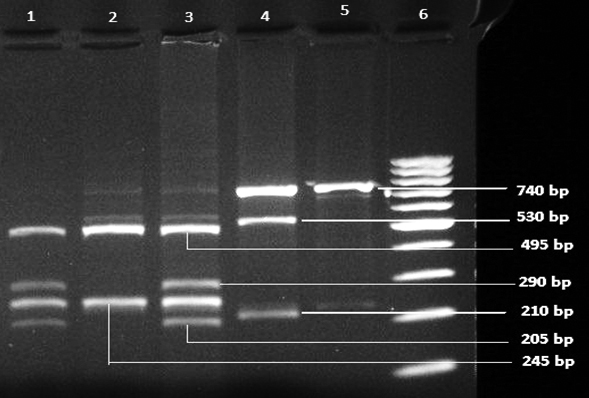
In case of TaqI polymorphism, the PCR product of 740 bp was obtained and digested with Taq-I restriction enzyme from NEB. Taq-I digestion revealed one obligatory restriction site, the homozygous TT (absence of the specific Taq-I restriction site) yielded bands of 245 bp and 495 bp. The homozygous tt exhibited 205, 245, 290 bp and the heterozygous Tt provided 495, 205, 245, 290 bp fragments.
For ApaI polymorphism, PCR product of 740 bp was digested with Apa-I restriction enzyme. The lack of ApaI site showed only one band at 740 bp and designated as AA, whereas presence of restriction site showed two fragments at 210 and 530 bp and designated as aa. The heterozygote displayed three fragments of 740, 210 and 530 designated as Aa [Table/Fig-1].
Statistical Analysis
Data was analysed using Graph Pad Prism 5.0 version. Group difference between ages was evaluated by unpaired t-test. Conformity towards Hardy-Weinberg equilibrium was calculated by the Chi-square test. Association between groups for genotypes and alleles were determined by contingency table analysed by calculating Odd’s ratio. A p-value <0.05 was considered as statistically significant.
Results
Age group analysis showed that 80% of the patients were above 60 years of age and 20% between the ages 51-60 years [Table/Fig-2].
Demographic characteristics of study subjects.
| Characteristics | Cases (N=120) | Control Group I (N=120) | Control Group II (N=120) |
|---|
| Mean age in years | 68.3 | 65.8 | 40.4 |
| Alcohol drinking |
| Yes | 52 (43.3%) | 44 (36.7%) | 48 (40%) |
| No | 68 (56.7%) | 76 (63.3%) | 72 (60%) |
| Smoking |
| Yes | 63 (52.5%) | 58 (48.3%) | 54 (45%) |
| No | 57 (47.5%) | 62 (51.7%) | 66 (55%) |
[Table/Fig-3] and [Table/Fig-4] shows the distribution of VDR gene polymorphism (TaqI, ApaI and FokI) in the study groups. Frequencies of single allele are shown in [Table/Fig-5]. Difference between single allelic frequencies was not significant. Distribution of genotypes by Chi-square test was carried out to find whether these genotypes are in accordance with Hardy-Weinberg Equilibrium. Comparison of VDR gene polymorphism between prostate cancer and control Group I (healthy non-related controls) showed that incidence of Tt and Aa genotype was more in controls as compared to cases and this was statistically significant [Table/Fig-3]. No significant association was found in FokI polymorphism. Single alleles were also compared between each group and no significant difference was found. Comparison of VDR gene polymorphism between prostate cancer and control Group II (first degree relatives of cases) was made [Table/Fig-4]. It shows higher incidence of Tt genotype in control Group II as compared to cases. None of the ApaI and FokI genotype was found to be statistically significant. Proportion of each single allele was also compared and no significant difference was found.
Comparison of VDR gene polymorphism between cases v/s control group I (healthy non-related controls).
| Polymorphism | Genotype and allele | Cases n (%) | Control Group I n (%) | odds Ratio | 95% CI | p-value |
|---|
| Taql | TT | 68 (56.7) | 40 (33.3) | 1 (ref | | |
| Tt | 36 (30) | 74 (61.7) | 0.286 | 0.108-0.758 | 0.016* |
| tt | 16 (13.3) | 6 (5) | 1.569 | 0.307-8.014 | 0.692 |
| Apal | AA | 56 (46.6) | 40 (33.4) | 1 (ref) | | |
| Aa | 32 (26.7) | 70 (58.3) | 0.336 | 0.120-0.941 | 0.043* |
| aa | 32 (26.7) | 10 (8.3) | 1.905 | 0.540-.715 | 0.355 |
| Fokl | FF | 64 (53.3) | 86 (71.7) | 1 (ref) | | |
| Ff | 52 (43.3) | 30 (25) | 2.329 | 0.911-5.956 | 0.091 |
| ff | 4 (3.3) | 4 (3.3) | 1.344 | 0.114-15.87 | 1.00 |
p<0 05
Comparison of VDR gene polymorphism between cases v/s control Group II (first degree relatives of cases).
| Polymorphism | Genotype | Cases n (%) | Control Group II n (%) | Odds Ratio | 95% CI | p-value |
|---|
| Taql | TT | 68 (56.6) | 28 (23.3) | 1 (ref) | | |
| Tt | 36 (30) | 84 (70) | 0.177 | 0.054-0.573 | 0.05* |
| Tt | 16 (13.4) | 8 (6.7) | 0.824 | 0.121-5.575 | 1.00 |
| Apal | AA | 56 (46.6) | 44 (36.7) | 1 (ref) | | |
| Aa | 32 (26.7) | 60 (50) | 0.419 | 0.131-1.345 | 0.160 |
| Aa | 32 (26.7) | 16 (13.3) | 1.571 | 0.373-6.613 | 0.723 |
| Fokl | FF | 64 (53.3) | 84 (70) | 1 (ref) | | |
| Ff | 52 (43.3) | 32 (26.7) | 2.133 | 0.713-6.376 | 0.274 |
| Ff | 4 (3.3) | 4 (3.3) | 1.313 | 0.0761-22.64 | 1.00 |
p<0.01
Distribution of allelic frequency among cases and controls.
| Polymorphism | Allele | Cases n (%) | Control Group In (%) | Control Group II n (%) |
|---|
| Taql | T | 172 (71.7) | 154 (64.2) | 140 (58.3) |
| t | 68 (28.3) | 86 (35.8) | 100 (41.7) |
| Apal | A | 144 (60) | 148 (61.7) | 148 (61.7) |
| a | 96 (40) | 92 (38.3) | 92 (38.3) |
| Fokl | F | 180 (75) | 202 (84.2) | 200 (83.3) |
| f | 60 (25) | 38 (15.8) | 40 (16.7) |
Thus, it was observed that Tt and Aa genotype was significantly low in prostate cancer patients when compared to healthy controls.
Frequency of Tt genotype was also low in prostate cancer patients as compared to relative control group. No significant association was found with FokI polymorphism.
Discussion
In the present study, we investigated the association of three VDR gene polymorphisms (FokI, TaqI and ApaI) with the development of prostate cancer. Our study revealed that persons having heterozygous allele, Tt and Aa have protection against the development of prostate cancer. However, we did not find association between prostate cancer and FokI polymorphism.
There is a large variation in the incidence rates of prostate cancer among racial ethnic groups worldwide. Incidence is high in Western world as compared to Asia [27]. The epidemiological data supports a major genetic component to prostate cancer risk but the studies associating VDR gene polymorphisms with the risk of development of prostate cancer has shown conflicting results.
Medeiros R et al., conducted a study in Portugal and found that both TT and Tt genotypes are over represented in prostate cancer patients [28]. In a study consisting of French and German men; the t allele was found to be the risk allele and linked with increase prostate cancer rates. In this case however, only the Tt genotype was found to be statistically significant in the risk of prostate cancer [29]. Taylor JA et al., claimed that men with the homozygous t allele had a one-third risk for developing prostate cancer compared with men who were heterozygotes or homozygotes for T allele [30]. In our study, we found that heterozygous Tt allele has protective role against the development of prostate cancer.
The study conducted in India by Mishra DK et al., found a higher incidence of FF genotype in patients as compared to controls (60.9 vs. 42.2 %). The frequency of ff genotype was significantly lower in cases (3.9%) while our study observed ff genotype in 3.3% of patients. They also opined that the f allele could be protective in nature and hence less aggressive [31].
Several authors have conducted meta-analysis of the published studies so far. A meta-analysis of 36 published studies carried out by Yin M et al., suggested that TaqI t allele was associated with reduced prostate cancer risk in overall population, whereas ApaI a allele was associated with reduced prostate cancer risk only in Asian population. In contrast, FokI f allele was associated with a trend of increased prostate cancer risk only in Caucasian population [32]. Zhang Q et al., performed a meta-analysis of 40 studies associating VDR gene polymorphism and prostate cancer and concluded that FF genotype had protective effect on prostate cancer in the Caucasian population. Conversely, TT genotype was associated with increased risk of prostate cancer whereas no significant association was found between ApaI gene polymorphism and prostate cancer risk [33]. A recent meta-analysis done by Wang K et al., demonstrated a non-significant association ApaI polymorphism with prostate cancer risk [34].
These conflicting reports may be due to the differences in the ethnicity, as it has been observed that polymorphisms having positive association in Asian population have little or no effect on prostate cancer risk in Caucasians and vice versa [35]. Based on the observations of the present study, it can be suggested that ‘Tt’ and ‘Aa’ genotypes might have a protective role against the development of prostate cancer. [Table/Fig-6] depicts different reports regarding VDR gene polymorphism and prostate cancer risk [28-31,36-41]. Although, TaqI and ApaI polymorphism does not seem to affect VDR amino acid sequence, their distribution may be relevant with VDR mRNA stability and gene transcription [42].
Worldwide studies on genetic variants of VDR and association with risk of prostate cancer [28-31,36-41].
| Sr. No. | Study | Country | Polymorphism | Association |
|---|
| 1. | Chokkalingam et al., [36] | China | FokI, BsmI | NS |
| 2. | Taylor JA et al., [30] | USA | TaqI | ’tt’ protective |
| 3. | Correa-Cerro L et al., [29] | Germany | TaqI | ’Tt’ protective |
| 4. | Medeiros R et al., [28] | Portugal | TaqI | T causative |
| 5. | Luscombe CJ et al., [37] | UK | FokI, TaqI | NS |
| 6. | Mishra DK et al., [31] | India | FokI | ’FF’ causative |
| 7. | Torkko KC et al., [38] | USA | FokI | NS |
| 8. | Bai Y et al., [39] | China | FokI, TaqI, ApaI | NS |
| 9. | Jingwi EY et al., [40] | USA | TaqI, ApaI, BsmI | TT and AA causative |
| 10. | Nunes HB et al. [41] | Brazil | FokI, TaqI, ApaI, BsmI | NS |
| 11. | Present study | India | FokI, TaqI, ApaI | ‘Tt’ and Aa’ protective |
NS: Not significant
This would result in alteration in the ability of VDR protein to bind 1,25(OH)2D3 or activate VDRE gene and results in changes in the expression of regulatory genes, such as CDK, which control prostatic cell division. The protective effect of Tt and Aa suggests that these genotypes may be less responsive to cell proliferation. But, further research is required including large cohort population of different race and ethnicity using more polymorphic sites to apply this finding for clinical application. This study can also be useful in near future for genetic screening of prostate cancer susceptibility.
Limitation
The limitation of the study is small sample size. A study on larger sample size is needed to further explore association of VDR gene polymorphism with development of prostate cancer.
Conclusion
This study indicated protective role of ‘Tt’ and ‘Aa’ genotypes against the development of prostate cancer in North Indian population while FokI polymorphism did not reveal any association.
*p<0 05
*p<0.01
NS: Not significant
[1]. Jemal A, Murray T, Samuels A, Ghafoor A, Ward E, Thun MJ, Cancer statistics 2003CA Cancer J Clin 2003 53(1):5-26. [Google Scholar]
[2]. Chatterjee A, Risk of prostate cancer in eastern IndiaInt J Cancer Res 2012 8(2):63-68. [Google Scholar]
[3]. Kamangar F, Dores GM, Anderson WF, Patterns of cancer incidence, mortality, and prevalence across five continents:defining priorities to reduce cancer disparities in different geographic regions of the worldJ Clin Oncol 2006 24(14):2137-50. [Google Scholar]
[4]. Schulz WA, Burchardt M, Cronauer MV, Molecular biology of prostate cancerMol Hum Reprod 2003 9:437-48. [Google Scholar]
[5]. Lessick M, Katz A, A genetics perspective on prostate cancerUrol Nurs 2006 26(6):454-60. [Google Scholar]
[6]. De Marzo AM, Marchi VL, Epstein JI, Nelson WG, Proliferative inflammatory atrophy of the prostate:implications for prostatic carcinogenesisAm J Pathol 1999 155(6):1985-92. [Google Scholar]
[7]. Nam RK, Zhang WW, Loblaw DA, Klotz LH, Trachtenberg J, Jewett MA, A genome-wide association screen identifies regions on chromosomes 1q25 and 7p21 as risk loci for sporadic prostate cancerProstate Cancer Prostatic Dis 2008 11(3):241-46. [Google Scholar]
[8]. Rajakumar K, Vitamin D, cod-liver oil, sunlight, and rickets:a historical perspectivePediatrics 2003 112(2):e132-35. [Google Scholar]
[9]. Dawson-Hughes B, Heaney RP, Holick MF, Lips P, Meunier PJ, Vieth R, Estimates of optimal vitamin D statusOsteoporos Int 2005 16(7):713-16. [Google Scholar]
[10]. Holick MF, Vitamin D deficiencyN Engl J Med 2007 357:266-81. [Google Scholar]
[11]. Bouillon R, Carmeliet G, Verlinden L, Van Etten E, Verstuyf A, Luderer HF, Vitamin D and human health:lessons from vitamin D receptor null miceEndocr Rev 2008 29(6):726-76. [Google Scholar]
[12]. Torkildsen Ø, Knappskog PM, Nyland HI, Myhr KM, Vitamin D-dependent rickets as a possible risk factor for multiple sclerosisArch Neurol 2008 65(6):809-11. [Google Scholar]
[13]. Schwartz GG, Whitlatch LW, Chen TC, Lokeshwar BL, Holick MF, Human prostate cells synthesize 1,25-dihydroxyvitamin D3 from 25-hydroxyvitamin D3Cancer Epidemiol Biomarkers Prev 1998 7(5):391-95. [Google Scholar]
[14]. Kivineva M, Bläuer M, Syvälä H, Tammela T, Tuohimaa P, Localization of 1,25-dihydroxyvitamin D3 receptor (VDR) expression in human prostateJ Steroid Biochem Mol Biol 1998 66(3):121-27. [Google Scholar]
[15]. Peehl DM, Skowronski RJ, Leung GK, Wong ST, Stamey TA, Feldman D, Antiproliferative effects of 1,25-dihydroxyvitamin D3 on primary cultures of human prostatic cellsCancer Res 1994 54(3):805-10. [Google Scholar]
[16]. Crescioli C, Maggie M, Vannelli GB, Luconi M, Salerno R, Barni T, Effect of a vitamin D3 analogue on keratinocyte growth factor-induced cell proliferation in benign prostate hyperplasiaJ Clin Endocrinol Metab 2000 85(7):2576-83. [Google Scholar]
[17]. Crescioli C, Ferruzzi P, Caporali A, Mancina R, Comerci A, Muratori M, Inhibition of spontaneous and androgen-induced prostate growth by a nonhypercalcemic calcitriol analogEndocrinology 2003 144(7):3046-57. [Google Scholar]
[18]. Taymans SE, Pack S, Pak E, Orban Z, Barsony J, Zhuang Z, The human vitamin D receptor gene (VDR) is localized to region 12cen-q12 by fluorescent in situ hybridization and radiation hybrid mapping:genetic and physical VDR mapJ Bone Miner Res 1999 14(7):1163-66. [Google Scholar]
[19]. Crofts LA, Hancock MS, Morrison NA, Eisman JA, Multiple promoters direct the tissue-specific expression of novel N-terminal variant human vitamin D receptor gene transcriptsProc Natl Acad Sci U S A 1998 95(18):10529-34. [Google Scholar]
[20]. Miyamoto K, Kesterson RA, Yamamoto H, Taketani Y, Nishiwaki E, Tatsumi S, Structural organization of the human vitamin D receptor chromosomal gene and its promoterMol Endocrinol 1997 11(8):1165-79. [Google Scholar]
[21]. Arai H, Miyamoto K, Taketani Y, Yamamoto H, Iemori Y, Morita K, A vitamin D receptor gene polymorphism in the translation initiation codon:effect on protein activity and relation to bone mineral density in Japanese womenJ Bone Miner Res 1997 12(6):915-21. [Google Scholar]
[22]. Hustmyer FG, DeLuca HF, Peacock M, ApaI, BsmI, EcoRV and TaqI polymorphisms at the human vitamin D receptor gene locus in Caucasians, blacks and AsiansHum Mol Genet 1993 2(4):487 [Google Scholar]
[23]. Fritz A, Ries L, The SEER program code manual 1998 3Available from: http://seer.cancer.gov/manuals/codeman.pdf [Google Scholar]
[24]. Daly AK, Steen VM, Fairbrother KS, Idle JR, CYP2D6 multiallelismMethods Enzymol 1996 272:199-210. [Google Scholar]
[25]. Harris SS, Eccleshall TR, Gross C, Dawson-Hughes B, Feldman D, The vitamin D receptor starts codon polymorphism (FokI) and bone mineral density in premenopausal American black and white womenJ Bone Miner Res 1997 12(7):1043-48. [Google Scholar]
[26]. Riggs BL, Nguyen TV, Melton LJ, 3rdMorrison NA, O’Fallon WM, Kelly PJ, The contribution of vitamin D receptor gene alleles to the determination of bone mineral density in normal and osteoporotic womenJ Bone Miner Res 1995 10(6):991-96. [Google Scholar]
[27]. Abouassaly R, Thompson IM, Platz EA, Klein EA, Kavoussi LR, Novick AC, Partin AW, Peters CA, Epidemiology, etiology, and prevention of prostate cancerCampbell–Walsh Urology 2012 10th editionPhiladelphiaElsevier Saunders:2690-2711. [Google Scholar]
[28]. Medeiros R, Morais A, Vasconcelos A, Costa S, Pinto D, Oliveira J, The role of vitamin D receptor gene polymorphisms in the susceptibility to prostate cancer of a southern European populationJ Hum Genet 2002 47(8):413-18. [Google Scholar]
[29]. Correa-Cerro L, Berthon P, Häussler J, Bochum S, Drelon E, Mangin P, Vitamin D receptor polymorphisms as markers in prostate cancerHum Genet 1999 105(3):281-87. [Google Scholar]
[30]. Taylor JA, Hirvonen A, Watson M, Pittman G, Mohler JL, Bell DA, Association of prostate cancer with vitamin D receptor gene polymorphismCancer Res 1996 56(18):4108-10. [Google Scholar]
[31]. Mishra DK, Bid HK, Srivastava DS, Mandhani A, Mittal RD, Association of vitamin D receptor gene polymorphism and risk of prostate cancer in IndiaUrol Int 2005 74(4):315-18. [Google Scholar]
[32]. Yin M, Wei S, Wei Q, Vitamin D receptor genetic polymorphisms and prostate cancer risk:a meta-analysis of 36 published studiesInt J Clin Exp Med 2009 2(2):159-75. [Google Scholar]
[33]. Zhang Q, Shan Y, Genetic polymorphisms of vitamin D receptor and the risk of prostate cancer:a meta-analysisJ Buon 2013 18(4):961-69. [Google Scholar]
[34]. Wang K, Wu G, Li J, Song W, Role of vitamin D receptor gene Cdx2 and Apa1 polymorphisms in prostate cancer susceptibility:a meta-analysisBMC Cancer 2016 16(1):674 [Google Scholar]
[35]. Dianat SS, Margreiter M, Eckersberger E, Finkelstein J, Kuehas F, Herwig R, Gene polymorphisms and prostate cancer:the evidenceBJU Int 2009 104(11):1560-72. [Google Scholar]
[36]. Chokkalingam AP, McGlynn KA, Gao YT, Pollak M, Deng J, Sesterhenn IA, Vitamin D receptor gene polymorphisms, insulin-like growth factors, and prostate cancer risk:a population-based case-control study in ChinaCancer Res 2001 61(11):4333-36. [Google Scholar]
[37]. Luscombe CJ, French ME, Liu S, Saxby MF, Jones PW, Fryer AA, Prostate cancer risk:associations with ultraviolet radiation, tyrosinase and melanocortin-1 receptor genotypesBr J Cancer 2001 85(10):1504-09. [Google Scholar]
[38]. Torkko KC, Van Bokhoven A, Mai P, Beuten J, Balic I, Byers TE, VDR and SRD5A2 polymorphisms combine to increase risk for prostate cancer in both non-Hispanic White and Hispanic White menClin Cancer Res 2008 14(10):3223-29. [Google Scholar]
[39]. Bai Y, Yu Y, Yu B, Ge J, Ji J, Lu H, Association of vitamin D receptor polymorphisms with the risk of prostate cancer in the Han population of Southern ChinaBMC Med Genet 2009 10:125 [Google Scholar]
[40]. Jingwi EY, Abbas M, Ricks-Santi L, Winchester D, Beyene D, Day A, Vitamin D receptor genetic polymorphisms are associated with PSA level, Gleason score and prostate cancer risk in African-American menAnticancer Res 2015 35(3):1549-58. [Google Scholar]
[41]. Nunes SB, De Matos Oliveira F, Neves AF, Araujo GR, Marangoni K, Goulart LR, Association of vitamin D receptor variants with clinical parameters in prostate cancerSpringerplus 2016 5:364 [Google Scholar]
[42]. Morrison NA, Qi JC, Tokita A, Kelly PJ, Crofts L, Nguyen TV, Prediction of bone density from vitamin D receptor allelesNature 1994 367(6460):284-87.Erratum in: Nature 1997;387(6628):106 [Google Scholar]