AVI is a disease that courses with the coexistence of volume and pressure overload. The eccentric hypertrophy, initially compensated, allows to the LV increase its compliance and filling pressure remains at normal levels, without overload to the left atrium. In the absence of pulmonary congestion, patients with AVI remain for long period without symptoms [1]. However, due to the adaptive mechanisms, important intrinsic changes occur in the myocardium and may induce cellular dysfunction, even in the absence of symptoms. In general, the diagnosis of cardiac dysfunction occurs late, with less possibility of regression of the structural abnormalities.
The major criteria of adverse prognosis in AVI are: presence of symptoms, LV dilation and LV systolic dysfunction. The role of LV diastolic dysfunction is not well established in the literature [2-4]. Regarding the myocardial hypertrophy, a change in the gene expression occurs, leading to an insufficient quantity or altered function in the proteins involved in calcium homeostasis. Therefore, a reduction in the calcium supply to the ventricular systole and additional supply calcium during diastole may occur, impairing the LV filling [5-7]. In addition, diastolic dysfunction is associated with unfavourable outcomes in various cardiac pathologies, such as arterial hypertension, coronary artery disease and aortic valve stenosis [8]. We did not find studies that associated more recent echocardiographic indices of diastolic function and adverse outcome predictors of AVI.
Considering the above, we hypothesize that diastolic dysfunction precede or coexist with systolic dysfunction in aortic insufficiency.
The aim of this study was to evaluate if echocardiographic indices of diastolic dysfunction are associated with unfavourable prognostic markers and severity criteria of AVI.
Materials and Methods
This was a prospective study including 22 consecutive patients diagnosed with moderate to severe AVI and referred from outpatient cardiology service of Botucatu Medical School-UNESP, Brazil from January 2012 to January 2013. All patients underwent a clinical evaluation and transthoracic echocardiography on the same day. This study was approved by ethics committee of Botucatu Medical School. Protocol 4043-2011.
The inclusion criteria were: age over 18 years and aortic valve insufficiency; moderate to severe. Exclusion criteria were: prosthetic heart valve; congestive heart failure due to other cause than AVI; other associated heart valves lesion greater than mild.
The criteria of unfavourable prognosis of AVI, according to the latest guidelines of the American Society of Cardiology [2,3] were: presence of angina, dyspnea or syncope in clinical evaluation, echocardiogram revelling reduced left ventricle ejection fraction (less than 0.5) and LV diastolic diameter greater than 60 mm. The criteria of the severity of aortic valve reflow, according to American Echocardiogram guidelines [3] were: moderate to severe aortic regurgitation assessed by Color Doppler, vena contracta between 0.3 to 0.6 cm for moderate reflow and greater than 0.6 cm for severe reflow, the presence of reverse holodiastolic flow in descending aorta and regurgitant jet occupying more than 50% of the LV outflow.
LV hypertrophy was defined as; LV mass indexed to body surface greater than 95 g/m2 in women and greater than 115 g/m2 in men [9].
The criteria of diastolic dysfunction with increased filling pressure were [8]: left atrium volume indexed to body surface area greater than 28 ml/m2; E/ E’ > 12 (E=Mitral inflow E wave velocity, and E’=early diastolic mitral annulus velocity, obtained by Tissue Doppler, considering the average between septal and lateral E’); septal E’< 8 cm/s and lateral E’< 10cm/S8.
Clinical Evaluation: Clinical and epidemiological data were obtained and cardiovascular risk factors were defined according to the current guidelines of cardiology [4,10-12].
Transthoracic echocardiogram: This was performed in all patients by the same examiner, in accordance with the standardization techniques recommended by the American Society of Echocardiography [9].
Statistical Analysis
Variables were expressed as means and standard deviations or by medians and interquartile ranges. Categorical variables were expressed as proportions. Associations between clinical, epidemiological and echocardiographic were evaluated by student t-test for normally distributed variables or Mann-Whitney test for non-normal distribution. Simple linear regression was used to compare continuous variables. Comparison between proportions was performed by Chi-square test. Level of significance was p <0.05.
Results
As shown in [Table/Fig-1], the analyses of the sample revealed no gender-related differences (p=0.20). The mean age of study population was SD 62.3±20 years. Most of the patients were Caucasian (p<0.001), with previous diagnosis of AH (p<0.039), normoglycaemic (p<0.001), non smokers (p<0.001) and without known coronary artery disease (p=0.020).
Baseline Characteristics.
| N = 22 | Medium ± SD or % |
|---|
| Age (years) | 62.3±20.0 |
| Sex (M/W) | 63.6/4.5 |
| Race (W/NW) | 95.5/7.9 |
| SAH (Yes/No) | 73.4/26.6 |
| DM (Yes/No) | 6.3/93.7 |
| Obesity (Yes/N) | 28.9/71.1 |
| Dyslipidemia (Yes/No) | 25.0/75.0 |
| Smoking (Yes/No) | 45.1/54.9 |
| CAD (Yes/No) | 20.0/80.0 |
| Severity of AVI (M/S) | 50.0/50.0 |
SD: Standard Deviation; M: Men; W: Women; W: White; NW: Non White; SAH: Systemic Arterial Hypertension; DM: Diabetes Mellitus; CAD: Coronary Artery Disease; AVI: Aortic valve insuf- ficiency; M: Moderate; S: Severe; P: significance level; *: p<0,05.
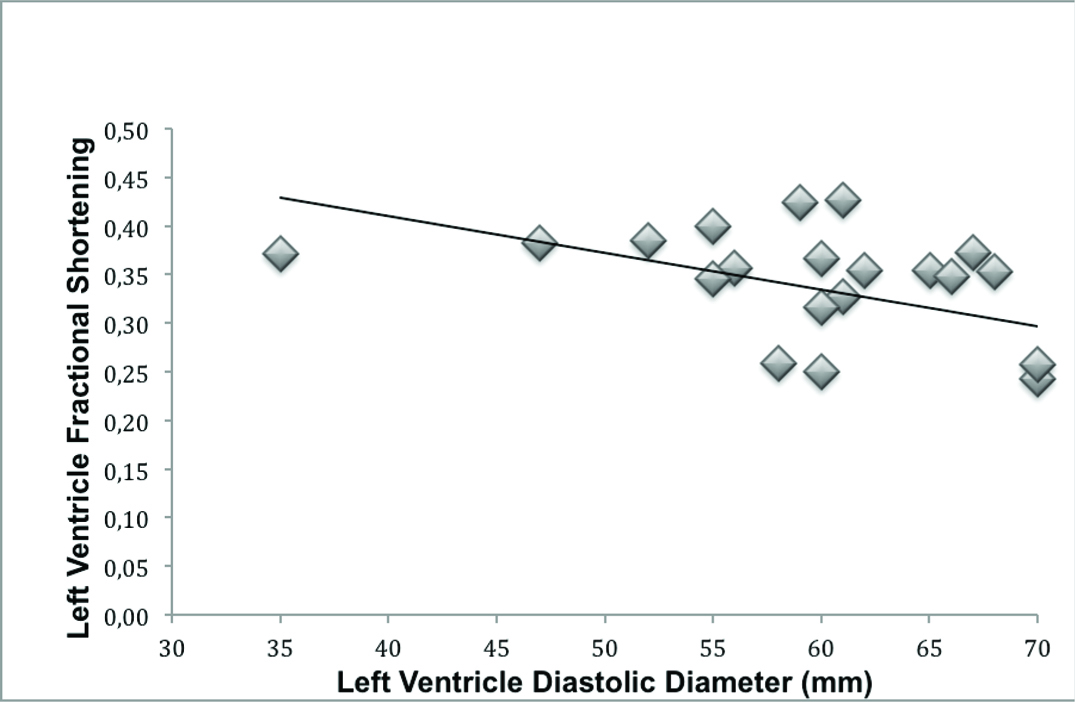
There was an inverse association between left ventricular dilation and decreased LV fractional shortening (R=0.51, R2=0.22, p= 0.016; [Table/Fig-2]) and a decrease in LV ejection fraction assessed by Simpson method (R= 0.42, R2= 0.14, p= 0.051). In addition, patients with worse LV fractional shortening had more echocardiographic criteria of severe AVI (p= 0.027).
Inverse association between left ventricle dilation and left ventricle systolic functional (linear regression: R = 0.51, R2 = 0.22, p = 0.016).
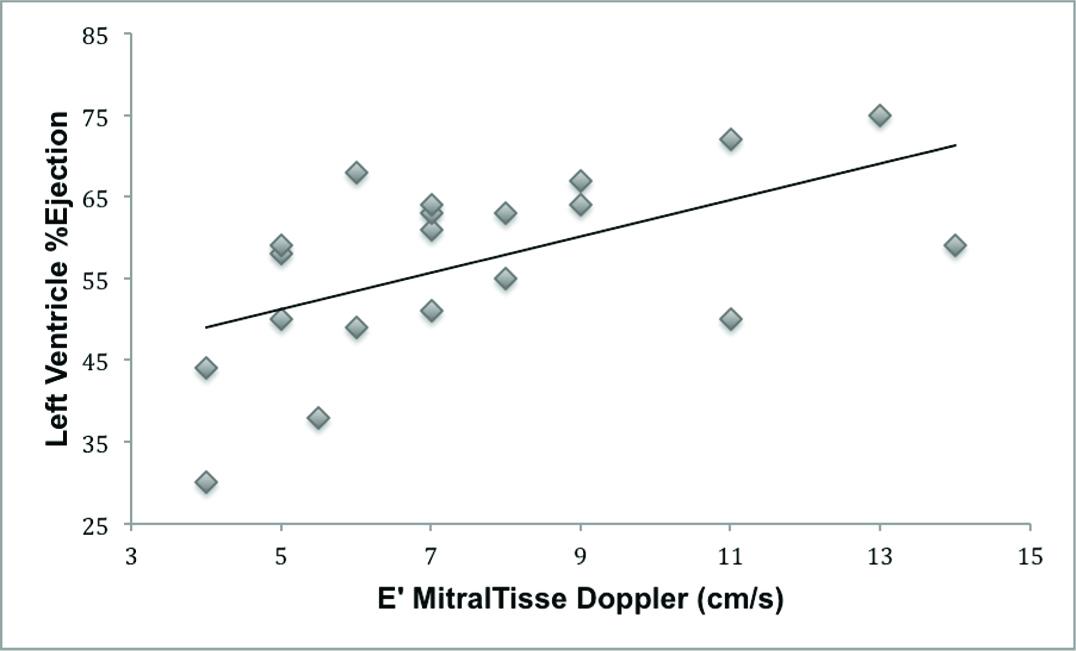
Regarding the assessment of diastolic function, there was no association between the E’ of mitral tissue Doppler with the following adverse outcome predictors and severity of AVI: ventricular dilation (R= 0.082, R2= 0.001, p= 0.724), the presence of symptoms of AVI (p= 0.713) and severity criteria of AVI (p=0,257). There was a significant association between E’ and the LV ejection fraction by Simpson method (R= 0.563, R2= 0.281, p= 0.008; [Table/Fig-3]) and marginal association with LV fractional shortening (R= 0.415, R2= 0.128, p= 0.062).
Association between E’ mitral tissue Doppler and left ventricle % ejection by Simpson method (linear regression: R = 0.56, R2 = 0.28, p = 0.008).
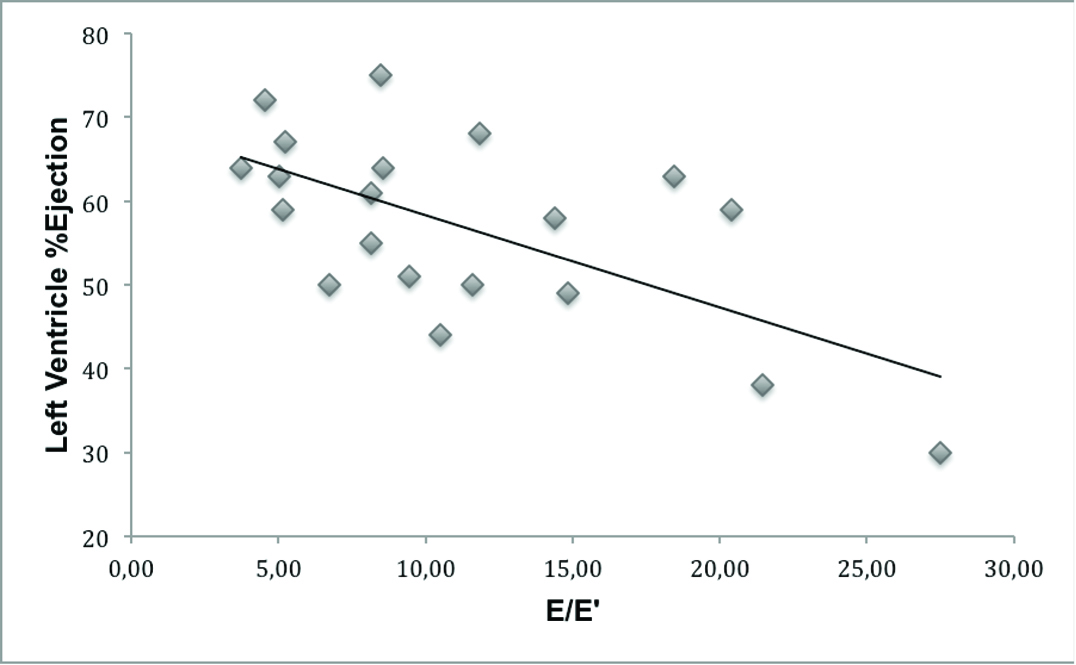
There was no association between mitral Doppler E/E’ with: ventricular dilation (R= 0.001, R2= 0.0001, p= 0.995), severity criteria of AVI (p= 0.107) and the presence of symptoms (p= 0.592). However, there was an inverse association between the E/E’ and LV ejection fraction (R= 0.639, R2= 0.378 and p= 0.002; [Table/Fig-4]) and marginal association with the LV fractional shortening (R= 0.415, R2= 0.129, p= 0.061).
Inverse association between E velocity of mitral inflow (E)/E’mitral tissue Doppler and left ventricle % ejection (linear regression: R = 0.639, R2 = 0.378 and p = 0.002).
The left atrium indexed volume was not associated with: ventricular dilation (R= 0.21, R2= 0.021, p= 0.29), severity criteria of the AVI (p= 0.314), presence of symptoms (p= 0.160), LV ejection fraction (R= 0.639, R2= 0.378, p= 0.002) and LV fractional shortening (R= 0.415, R2= 0.129, p= 0.061).
Left ventricular mass indexed to body surface was inversely associated with the LV ejection fraction by Simpson (R= 0.62, R2= 0.35, p= 0.003) and the LV fractional shortening (R= 0.65, R2= 0.40, p= 0.001).
Left ventricular indexed mass had no association with diastolic dysfunction criteria: Left atrial indexed volume (R= 0.061, R2= 0.000, p= 0.803), mitral tissue doppler E’ (R= 0.038, R2= 0.000, p= 0.87) and E/E’ (R= 0.015, R2= 0.000, p= 0.95).
Discussion
It is already known that AVI leads to eccentric hypertrophy secondary to volume overload, causing ventricular dilatation, change in geometry and increased ventricular compliance [5-7]. Patients remain asymptomatic for decades. Nevertheless, these features provide late diagnosis of the severity of the disease, with the establishment of intrinsic myocardial damage and poor prognosis [1-3].
Therefore, it is worthy to study other echocardiographic criteria associated with severity of the disease.
A recent study showed that rats with experimental acute AVI were adapted with eccentric hypertrophy and ventricular spherical geometry without clinical signs of heart failure for 16 weeks [13]. The sphericity index increased the value of prediction of ventricular systolic dysfunction, considered important criterion of gravity and unfavourable evolution of the disease. In addition, there was an increase in left atrium area, considered marker of diastolic dysfunction, and this preceded the decrease of left ventricular fractional shortening.
Our research work showed an association of a more recent echocardiographic criteria of diastolic dysfunction with a decrease in systolic function [Table/Fig-3,4], suggesting that patients with moderate or severe AVI present signs of increased ventricular filling pressure and diastolic dysfunction may coexist with or even preced the myocardial dysfunction. There was no association between diastolic dysfunction and ventricular dilation, suggesting that the left ventricle volume is not only responsible for the increase of filling pressure. De Gobbi et al., observed similar results studying antidepressant treatment in a model of aortic regurgitation in rats [14].
There was an association between left ventricle mass with reduced systolic function, suggesting that the LV hypertrophy may be another index associated with severity criteria of the disease. On the other hand, the increase in ventricular mass was not associated with diastolic dysfunction criteria, suggesting that other mechanisms also contribute to the increase in ventricular filling pressure in these patients.
The limitations of this study were the small number of participants. Aortic regurgitation is not a common disease and under diagnosed, because of absence of symptoms for a long time. In addition, many symptomatic patients underwent urgently valve replacement and they were not included in this study.
The importance of our study is the correlation between diastolic dysfunction and impaired ventricular function, suggesting that diastolic function obtained by echocardiography should also be considered in AVI patients. The left ventricular hypertrophy also was correlated with the severity of AVI and ventricular dysfunction and should also be considered in the management of these patients.
Conclusion
The LV increased filling pressure and LV indexed mass is associated with severity of AVI, suggesting that LV diastolic function and degree of hypertrophy should also be considered in the follow up of AVI patients.
Financial Support: São Paulo Research Foundation - FAPESP Process: 2015/00275-5.
SD: Standard Deviation; M: Men; W: Women; W: White; NW: Non White; SAH: Systemic Arterial Hypertension; DM: Diabetes Mellitus; CAD: Coronary Artery Disease; AVI: Aortic valve insuf- ficiency; M: Moderate; S: Severe; P: significance level; *: p<0,05.