Autism Spectrum Disorder (ASD) disease has become a mounting socio-economical alarm around the world. Neuroinflammtion had been shown in postmortem brain specimens from ASD patients. The Endocannabinoids System (ES) consists of a family of locally produced, short-lived, endogenous, phospholipid-derived agonists (endocannabinoids) that control energy balance and body composition. The growing number of medical benefits of ES, such as their ability to regulate processes like neuroinflammation, neurogenesis and memory, raise the question of their potential role as a preventive treatment of ASD.
To test this hypothesis, basic and clinical studies allow us a thorough investigation of the role of ES in the pathogenesis of ASD. This hypothesis will help to understand the mechanism of ES and its role in ASD.
Introduction
ASD is a severe neurodevelopmental disorder characterized by repetitive, stereotyped behavior, impai-rment in communication and social interaction in children [1].
The number of cases in the Arab world ranges from 1.4 to 29 per 10000 [2]. While these rates are much lower than those found in the literature, it does not mean the condition is less prevalent in the Arab world, because many cases may remain undiagnosed. In fact, evidence does not support differences in ASD prevalence by geographic region nor of a strong impact of ethnic/cultural factors [1]. Factors that might contribute to an apparent lower prevalence of autism in Arab countries include low awareness of ASD in professionals (e.g., paediatricians) and in the general population (e.g., parents, teachers), few child neuro-psychiatrists in general and fewer specialized in ASD.
Although the diagnosis is made during first three years of age, however the onset of disease can be from birth or usually during the second year [3]. The aetiology of ASD is not fully understood but there is consensus that genetics, environmental and autoimmunity factors interact to play a role in its development [4]. Neuroinflammtion in ASD has attracted the attention of research in targeting the neuroprotective mechanisms [5]. The growing number of medical benefits of Endocannabinoid System (ES), such as their ability to regulate neuroinflammation, neurogenesis and memory, raise the question of their potential role as a preventive treatment of ASD. This hypothesis paper highlights the basic and clinical studies that allow us a thorough investigation of the role of ES in the pathogenesis of ASD [6].
Neuroinflammtion in ASD
Neuroinflammtion is an important aspect in ASD and had been shown in postmortem brain specimens from ASD patients of different age (4–45 years of age) groups [5]. Microglia, resident mononuclear cells of the CNS, takes part in immune surveillance and synaptic pruning [7]. Studies have shown prominent microglia activation and increased inflammatory cytokine and chemokine production, including IFN-γ, IL-1β, IL-6, IL-12p40, TNF-α (tumor necrosis factor α) and chemokine CCL-2 in the brain tissue and cerebral spinal fluid [8-10].
Expression profiling analysis of postmortem brain tissues from ASD patients revealed increased levels of mRNA transcripts of several immune-related genes [11]. In addition to neuroinflammation, there are alterations in the systemic immune responses that are associated with disease severity. For example, different proinflammatory cytokines such as, IL-1β, IL-6, IL-8, IL-12p40, and chemokines CCL2, CCL5, are elevated in ASD and are associated with poor communication and social interaction [12-14]. These findings indicate a trend towards neural and systemic proinflammatory status of the immune system in ASD.
Endocannabinoid system as neuro protective mechanisms
Central nervous system (CNS) has inherent mechanisms of neural cells protection. Activation of ES may be one of those mechanisms which are involved in protecting the negative effects of inflammation, because of its anti-inflammatory effects on CNS [15]. The ES is a novel system of intracellular signalling and consists of two main cannabinoid (CB) receptors type 1 and type 2 (CB1 and CB2). Initially identified in mouse spleen cells, CB1 receptors are located in the CNS, peripheral nervous system, and peripheral organs. CB1 receptors are also found in adipocytes, liver, pancreas and skeletal muscle. CB2 receptors are expressed on immune cells such as microglia (resident mononuclear cells of the immune system in CNS) and neurons. In the CNS, CB1 receptors are concentrated in the cerebellum, hippocampus, and the basal ganglia [16], which are areas in the brain implicated as dysfunctional in autism [17,18].
Linking endocannabinoid system, neuroinflammation and neurodegeneration
Although ES and the immune system are autonomous, however both systems communicate with each other anatomically and physiologically through autonomic nervous system via innervations of lymph nodes, spleen, bone marrow, thymus, liver and gastrointestinal tract. The two systems communicate through chemical messengers, which range from smaller molecules, including nitric oxide, to larger proteins, such as cytokines. Inflammation is one of the example which shows an intimate interaction between the two system acting in a complex manner to generate appropriate adaptive cellular responses [19]. Neuronal wiring of the ANS (sympathetic and parasympathetic) to the main sites of immune system such as thymus, liver, spleen and bone marrow, skin and lymph nodes modulates the immune system. Second, small molecules such as nitric oxide and large like cytokines and growth factors and their respective receptors connect these two systems. Thus, these two important entities of the body are interlinked physiologically and anatomically to mount an effective response when required [6].
Potential pro-inflammatory role of cannabinoids have also been reported in a few studies, indicating involvement of complex mechanisms that need to be unraveled [20,21].
It is well known that pro-inflammatory cytokines such as IL-6, IL-12, IL-1β, TNF-α released by immune cells in the CNS adds to the development of neuroinflammation and neurodegeneration [22]. Activation of ES is one the mechanism in the CNS, which protect from the detrimental effects of these cytokines. During inflammation, endogenous endocannabinoids such as Arachidonyl Ethanolamide (AEA) and 2-arachidonyl glycerol (2-AG) are released from different immune cells and neurons in the CNS. These endocannabinoid ligands (AEA, 2-AG) bind to endocannabinoids receptors and induce neuroprotection through different mechanisms [23].
Thus, it is possible that participation of ES in the regulation of immune responses is associated with therapeutic effects mediated by the down regulation of cytokine expression.
Regardless of the challenges in targeting ES receptors that will potentially disrupt the processes of learning and memory, approaches for neuroprotection have been taken to avoid those side effects by targeting specifically the ES CB2 receptors [Table/Fig-1], by modulation of the degradation pathway of ES, or by using low, non-psychoactive doses of non-selective agonists of CB1/CB2 endocannabinoid receptors [24].
Schematic representation of multisite actions of endocannabinoid system.
*Potential therapeutic target to treat neuroinflammation in autism through selective CB2 receptor agonists.
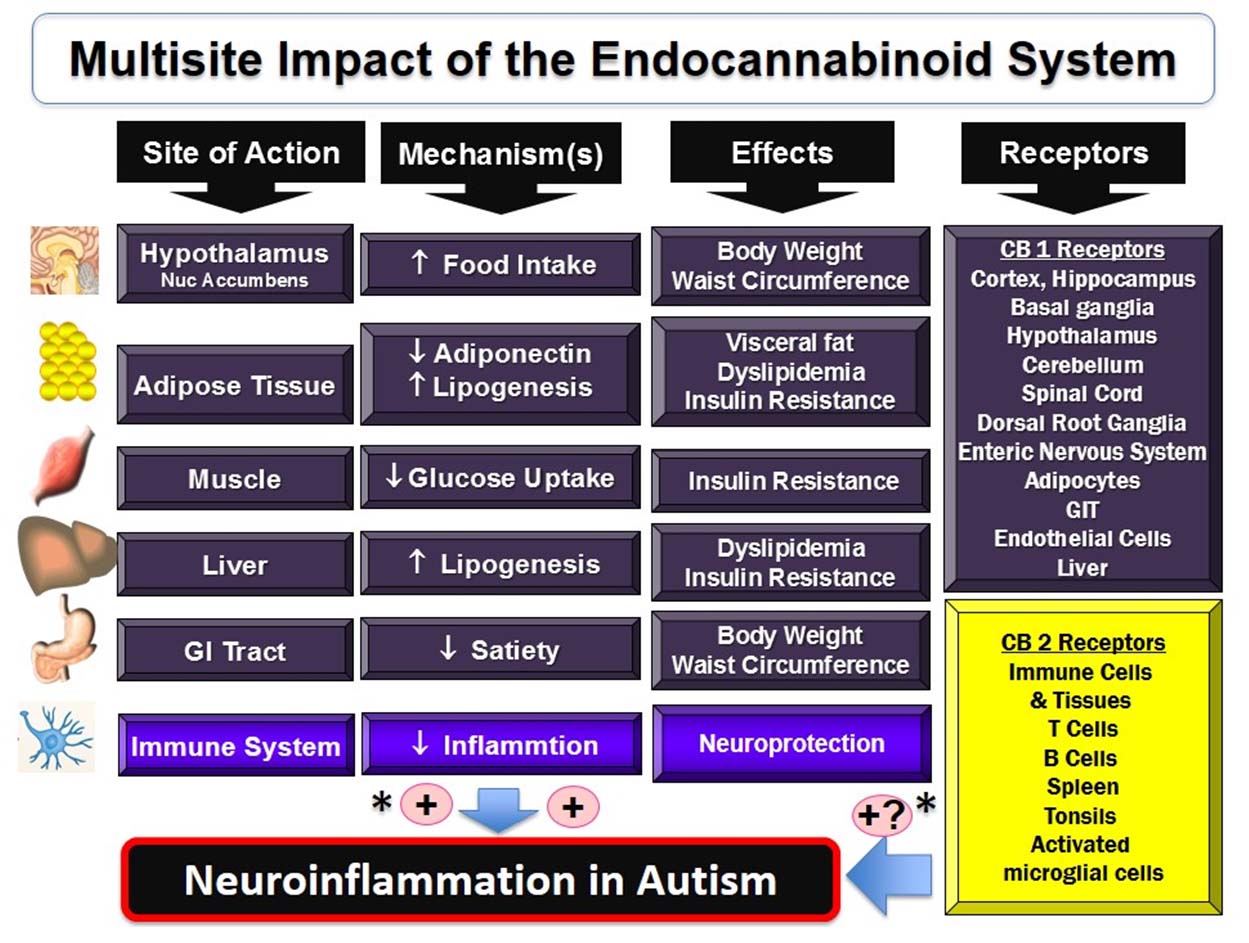
It has been shown that mRNA and protein levels of CB2 receptor were up-regulated in the blood, peripheral blood mononuclear cells, of children with autistic disorder [25], suggesting the involvement of the endocannabonoid system in the development of autism.
The ES plays an important role in the development of the central nervous system and its activation can induce long-lasting functional adaptations [26]. Use of cannabis (an exogenous cannabinoid) in the still-maturing brain may produce persistent alterations in brain structure and cognition [27]. Due to poor efficacy of current treatments and the likely delay to implement future safe and efficacious treatments, there is an opportunity to develop preventive approaches based on currently available knowledge regarding the pathogenesis and risk factors of ASD. It has been found that endocannabinoids have anti-inflammatory and immuno-suppressive effects and can act as potential therapies [28]. Different pharmacological agents providing anti-inflammatory and neuroprotective effects in the diseases of CNS can modulate Endocannabinoid receptors CB1, CB2 and other EC receptors which are not fully defined. For example, in experimental models of AD, stimulation of CB1, CB2 and other non-CB1 and non-CB2 receptors with cannabinoids reduced microglial activation and microglia-mediated neurodegeneration [29]. It has been shown that increase in endogenous levels of endocannabinids has protective effect against β-amyloid-induced neurotoxicity [30]. In experimental models of multiple sclerosis, stimulation of CBI and CB2 receptors have anti-inflammatory effects in response to pharmacological AMT inhibitors. AMT is a purported high-affinity transporter which is responsible for removal of anandamide and CB receptors and AMT inhibitors like AM404, VDM11 and UCM707 block cellular uptake of anandamide and CB receptors [31,32]. This therapeutic use of drugs targeting EC system in CNS disorders has a great potential and animal experiments have shown encouraging results in reducing clinical symptoms in degenerative and inflammatory disease conditions. For example AMT inhibitors such as AM404 and VDM11 showed anti-cytotoxic effects in experimental model of Parkinson’s disease. Similarly administration of AM404 and VD11 also reduced the spasticity in mice suffering from chronic relapsing experimental allergic encephalomyelitis, a model of MS [33].
Testing the hypothesis
Hence, ES modulators can be explored in autism animal models with well-established face and predictive validities which could serve as a translational link between laboratory findings from animal’s studies leading to exploratory studies in humans. Thus, we postulate that modulation of the ES in ASD could prove a valuable tool to prevent or delay the progression of disease [Table/Fig-1].
Implications of the hypothesis
The complex nature of ASD advocates a multimodal drug approach that could protect from the various processes underlying neurodegeneration and thus, at minimum, delay the pathological process. The expected benefit from a chronic treatment aimed at stimulating the endocannabinoid system is a delayed progression of ASD: i.e., reduced inflammation, sustained potential for neurogenesis, and delayed memory impairment. Such results could lead to new therapeutic strategies that target the inflammation and the decline in neurogenesis associated ASD.
Authors’ contributions
SH, MI, KA and SB participated to the conception of the present hypothesis. All authors drafted and approved the final manuscript.
[1]. Elsabbagh M, Divan G, Koh YJ, Kim YS, Kauchali S, Marcín C, Global prevalence of autism and other pervasive developmental disordersAutism Res 2012 5(3):160-79. [Google Scholar]
[2]. Al-Salehi SM, Al-Hifthy EH, Ghaziuddin M, Autism in Saudi Arabia:presentation, clinical correlates and comorbidityTranscult Psychiatry 2009 46(2):340-47. [Google Scholar]
[3]. Ozonoff S, Iosif AM, Baguio F, Cook IC, Hill MM, Hutman T, A prospective study of the emergence of early behavioral signs of autismJ Am Acad Child Adolesc Psychiatry 2010 49(3):256-66.e1-2 [Google Scholar]
[4]. Careaga M, Van de Water J, Ashwood P, Immune dysfunction in autism:a pathway to treatmentNeurotherapeutics 2010 7(3):283-92. [Google Scholar]
[5]. Vargas DL, Nascimbene C, Krishnan C, Zimmerman AW, Pardo CA, Neuroglial activation and neuroinflammation in the brain of patients with autismAnn Neurol 2005 57(1):67-81. [Google Scholar]
[6]. Ziemssen T, Kern S, Psychoneuroimmunology – cross-talk between the immune and nervous systemsJ Neurol 2007 254:8-11. [Google Scholar]
[7]. Bessis A, Béchade C, Bernard D, Roumier A, Microglial control of neuronal death and synaptic propertiesGlia 2007 55(3):233-38. [Google Scholar]
[8]. Li X, Chauhan A, Sheikh AM, Patil S, Chauhan V, Li XM, Elevated immune response in the brain of autistic patientsJ Neuroimmunol 2009 207(1-2):111-16. [Google Scholar]
[9]. Morgan JT, Barger N, Amaral DG, Schumann CM, Stereological study of amygdala glial populations in adolescents and adults with autism spectrum disorderPLoS ONE 2014 9(10):e110356 [Google Scholar]
[10]. Morgan JT, Chana G, Pardo CA, Achim C, Semendeferi K, Buckwalter J, Microglial activation and increased microglial density observed in the dorsolateral prefrontal cortex in autismBiol Psychiatry 2010 68(4):368-76. [Google Scholar]
[11]. Garbett K, Ebert PJ, Mitchell A, Lintas C, Manzi B, Mirnics K, Immune transcriptome alterations in the temporal cortex of subjects with autismNeurobiol Dis 2008 30:303-11. [Google Scholar]
[12]. Mead J, Ashwood P, Evidence supporting an altered immune response in ASDImmunol Lett 2015 163(1):49-55. [Google Scholar]
[13]. Xu N, Li X, Zhong Y, Inflammatory cytokines:potential biomarkers of immunologic dysfunction in autism spectrum disordersMediators Inflamm 2015 2015:531518 [Google Scholar]
[14]. Careaga M, Ashwood P, Autism spectrum disorders:from immunity to behaviorMethods Mol Biol 2012 934:219-40. [Google Scholar]
[15]. Micale V, Mazzola C, Drago F, Endocannabinoids and neurodegenerative diseasesPharmacol Res 2007 56:382-92. [Google Scholar]
[16]. Drysdale AJ, Platt B, Cannabinoids:mechanisms and therapeutic applications in the CNSCurr Med Chem 2003 10(24):2719-32. [Google Scholar]
[17]. Bauman ML, Kemper TL, Neuroanatomic observations of the brain in autism:a review and future directionsInt J Dev Neurosci 2005 23(2-3):183-87. [Google Scholar]
[18]. Courchesne E, Pierce K, Schumann CM, Redcay E, Buckwalter JA, Kennedy DP, Mapping early brain development in autismNeuron 2007 56(2):399-413. [Google Scholar]
[19]. Steinman L, Elaborate interactions between the immune and nervous systemsNat Immunol 2004 5:575-81. [Google Scholar]
[20]. Killestein J, Hoogervorst EL, Reif M, Blauw B, Smits M, Uitdehaag BM, Immunomodulatory effects of orally dministered cannabinoids in multiple sclerosisJ Neuroimmunol 2003 137(1-2):140-43. [Google Scholar]
[21]. Maestroni GJ, The endogenous cannabinoid 2-arachidonoyl glycerol as in vivo chemoattractant for dendritic cells and adjuvant for Th1 response to a soluble proteinFASEB J 2004 18(15):1914-16. [Google Scholar]
[22]. Wong ML, Sternberg EM, Immunological assays for understanding neuroimmune interactionsArch Neurol 2000 57:948-52. [Google Scholar]
[23]. Jean-Gilles L, Gran B, Constantinescu CS, Interaction between cytokines, cannabinoids and the nervous systemImmunobiology 2010 215(8):606-10. [Google Scholar]
[24]. Centonze D, Finazzi-Agrò A, Bernardi G, Maccarrone M, The endocannabinoid system in targeting inflammatory neurodegenerative diseasesTrends Pharmacol Sci 2007 28(4):180-87. [Google Scholar]
[25]. Siniscalco D, Sapone A, Giordano C, Cirillo A, de Magistris L, Rossi F, Cannabinoid receptor type 2, but not type 1, is up-regulated in peripheral blood mononuclear cells of children affected by autistic disordersJ Autism Dev Disord 2013 43:2686-95. [Google Scholar]
[26]. Campolongo P, Trezza V, Palmery M, Trabace L, Cuomo V, Developmental exposure to cannabinoids causes subtle and enduring neurofunctional alterationsInt Rev Neurobiol 2009 85:117-33. [Google Scholar]
[27]. Jager G, Ramsey NF, Long-term consequences of adolescent cannabis exposure on the development of cognition, brain structure and function:an overview of animal and human researchCurr Drug Abuse Rev 2008 1(2):114-23. [Google Scholar]
[28]. Nagarkatti P, Pandey R, Rieder SA, Hegde VL, Nagarkatti M, Cannabinoids as novel anti-inflammatory drugsFuture Med Chem 2009 1(7):1333-49. [Google Scholar]
[29]. Ramírez BG, Blázquez C, Gómez del Pulgar T, Guzmán M, de Ceballos ML, Prevention of Alzheimer’s disease pathology by cannabinoids:neuroprotection mediated by blockade of microglial activationJ Neurosci 2005 25(8):1904-13. [Google Scholar]
[30]. Van der Stelt M, Mazzola C, Esposito G, Matias I, Petrosino S, De Filippis D, Endocannabinoids and beta-amyloid-induced neurotoxicity in vivo:effect of pharmacological elevation of endocannabinoid levelsCell Mol Life Sci 2006 63(12):1410-24. [Google Scholar]
[31]. Arévalo-Martín A, Vela JM, Molina-Holgado E, Borrell J, Guaza C, Therapeutic action of cannabinoids in a murine model of multiple sclerosisJ Neurosci 2003 23(7):2511-16. [Google Scholar]
[32]. Eljaschewitsch E, Witting A, Mawrin C, Lee T, Schmidt PM, Wolf S, The endocannabinoid anandamide protects neurons during CNS inflammation by induction of MKP-1 in microglial cellsNeuron 2006 49(1):67-79. [Google Scholar]
[33]. Högestätt ED, Jönsson BA, Ermund A, Andersson DA, Björk H, Alexander JP, Conversion of acetaminophen to the bioactive N-acylphenolamine AM404 via fatty acid amide hydrolase-dependent arachidonic acid conjugation in the nervous systemJ Biol Chem 2005 280(36):31405-12. [Google Scholar]