OA is a chronic degenerative condition affecting various soft and hard tissues around the joint. Almost 15% of world’s population and 22-29% of Indian population is suffering from OA. A 18% of women and 9.6% of men with 60 years of age have symptomatic OA [1]. Mechanical factor is the main risk factor for initiation of disease progression. Other factors involved in OA process initiation are endogenous factors like mutation in type II collagen, or dysplastic conditions [2]. OA is characterized by continuous inflammatory response of cartilage of the articular surface resulting in erosion of cartilage and an increased osteoblastic activity or reparative bone response [3]. OA is a prevalent pathological condition of the TMJ. Osseous changes of TMJ OA include erosion, sclerosis, flattening, osteophyte formation, resorption of condylar head and joint space narrowing [4]. OA can cause clinical symptoms like pain, joint stiffness and muscle weakness [3]. Diclofenac is one of the often-used NSAID for management of pain because of its better tolerability [5]. The oral enzymes preparation containing bromelain, rutoside trihydrate and trypsin is suggested to have analgesic, anti-inflammatory, anti-edematous and antioxidant properties [5,6]. The existing combination of oral enzymes and diclofenac is easily available and cost effective, so a study was planned with the aim of comparing the effectiveness of oral bromelain, trypsin and rutoside trihydrate enzymes with diclofenac sodium combination therapy over diclofenac sodium and oral enzymes for treatment of OA of TMJ.
Materials and Methods
A randomised clinical trial was conducted in the Department of Oral Medicine and Radiology, Tamil Nadu Government Dental College and Hospital, Chennai after getting clearance from the ethical committee of the institute. Patients were included into the study after obtaining an informed consent. Based on the prevalence of number of patients reported, 30 patients in age group of 40 years to 60 years of both genders, diagnosed for TMJ OA were randomly divided into three groups for the purpose of treatment by simple random sampling. Group 1 (n=10) patients were treated with tablet diclofenac sodium 50 mg twice daily for 10 days. Group 2 (n=10) patients were given combination of bromelain 90 mg, rutoside trihydrate 100mg, trypsin 48 mg and diclofenac sodium 50 mg (FLAMAR 3D, SYNOKEM Pharmaceuticals Ltd., Haridwar, India) twice daily for 10 days. Group 3 (n=10) patients were given oral enzymes of bromelain 90 mg, rutoside trihydrate 100 mg, trypsin 48 mg (Rutoheal, Pharma Force Lab, Gondpur, India) twice daily for 10 days [Table/Fig-1]. Inclusion criteria for patients were clinical characteristics for TMJ OA such as pain at rest or mandibular movement, crepitation, limitation of mouth opening which were described by Okeson JP [7], followed by radiographic investigation for evidence of osseous changes at TMJ to confirm the diagnosis. Patient with myogenous cause of pain, ankylosis, recent history of any trauma or surgery at TMJ, history of peptic ulcer, drug allergy and pregnant women were excluded from the study.
Flow chart showing sample selection following inclusion and exclusion criteria and their division into various groups.
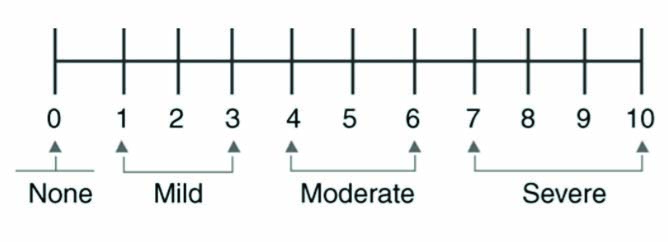
The pain scoring was recorded according to Numeric Rating Scale (NRS) [8], using numbers from 0 to 10 (‘no pain’ to ‘severe pain’) [Table/Fig-2]. Blinding was not carried out in the present study. Statistical analysis was performed using ANOVA followed by post-hoc test to compare pre and post treatment results, p<0.05 was considered as significant.
Numeric rating scale used for pain scoring from numbers 0 to 10.
Results
Within the 10 days observation period, all patients tolerated drugs uneventfully and came for regular follow up. Out of 30 patients in the study, there were 13 (43.3%) males and 17 (56.7%) females. The average age was found to be 49 years. Minimum age of the patient in the study was 41 and maximum age was 60 years. A 73.3% of patients complained of pain in one TMJ while 26.7% complained of pain in both TMJ. Starting from day 1 numerical rating scale score of 7.83±1.577 (Mean ± SD), all groups showed a decrease in pain on day 4 with mean of 5.07±2.273 and further decrease of pain on day 7 with mean of 2.93±2.132 and final mean of 2.07±1.818 on day 10.
As compared to mean of NRS score of 8.8 and 7.7 which reduced to 6.8 and 5.9 in group 1 and group 3 patients respectively, the remarkable decrease in mean from 7 to 2.5 was seen in group 2 patients when followed after 3 days. At day 7, the largest mean reduction to .40 was seen in group 2. Mean value of NRS score reduced to 4.50 and 3.90 for group 1 and group 3 respectively on days 7. Further reduction in pain score was seen on next visit showing clear improvement in treatment for all groups [Table/Fig-3].
Showing distribution of study groups according to mean and standard deviation in numeric rating scoring on day 1,4,7 and 10.
| Groups | N | Mean | Std. Deviation |
|---|
| Day 1 | Group 1 | 10 | 8.80 | 1.229 |
| Group 2 | 10 | 7.00 | 1.563 |
| Group 3 | 10 | 7.70 | 1.494 |
| Day 4 | Group 1 | 10 | 6.80 | 1.033 |
| Group 2 | 10 | 2.50 | 1.581 |
| Group 3 | 10 | 5.90 | 1.287 |
| Day 7 | Group 1 | 10 | 4.50 | 1.354 |
| Group 2 | 10 | 0.40 | 0.516 |
| Group 3 | 10 | 3.90 | 1.287 |
| Day 10 | Group 1 | 10 | 3.40 | 1.350 |
| Group 2 | 10 | 0.00 | 0.000 |
| Group 3 | 10 | 2.80 | 1.229 |
The one-way ANOVA was applied [Table/Fig-4] followed by Dunnett–t post-hoc [Table/Fig-5] and Games-Howell’s post-hoc test [Table/Fig-6] to test significant differences between the groups for analysing the efficacy of drugs in the management of TMJ OA during the course of treatment. p < 0.05 was considered statistically significant. This study demonstrated p < 0.05 showing statistically significant difference between group 2 and other two groups, which indicated that patients treated with oral bromelain, trypsin, rutoside trihydrate enzymes and diclofenac sodium combination therapy responded better than patients given diclofenac or oral enzymes preparation alone, while p > 0.05 between group 1 and group 3, revealed no significant differences between patients administered diclofenac or oral enzymes preparation.
Evaluation of effect of drugs on pain scoring using ANOVA.
| Variable | Sum of Squares | Degree of freedom | Mean Square | F value | p-value |
|---|
| Day 1 | Between GroupsWithin Groups | 16.46755.700 | 2 27 | 8.2332.063 | 3.991 | 0.030 |
| Day 4 | Between GroupsWithin Groups | 102.86747.000 | 2 27 | 51.4331.741 | 29.547 | 0.000 |
| Day 7 | Between GroupsWithin Groups | 98.06733.800 | 2 27 | 49.0331.252 | 39.169 | 0.000 |
| Day 10 | Between GroupsWithin Groups | 65.86730.000 | 2 27 | 32.9331.111 | 29.640 | 0.000 |
Comparison (p-value) between groups for pain assessment by Dunnett -t post-hoc test.
| Dependent Variables | Mean Difference | Sig. |
|---|
| Day 1 | Group 1 | Group 2 | 1.800 | 0.028 |
| Group 3 | 1.100 | 0.200 |
| Group 2 | Group 1 | -1.800 | 0.028 |
| Group 3 | -0.700 | 0.572 |
| Group 3 | Group 1 | -1.100 | 0.200 |
| Group 2 | 0.700 | 0.572 |
| Day 4 | Group 1 | Group 2 | 4.300 | 0.000 |
| Group 3 | 0.900 | 0.225 |
| Group 2 | Group 1 | -4.300 | 0.000 |
| Group 3 | -3.400 | 0.000 |
| Group 3 | Group 1 | -0.900 | 0.225 |
| Group 2 | 3.400 | 0.000 |
| Day 7 | Group 1 | Group 2 | 4.100 | 0.000 |
| Group 3 | 0.600 | 0.577 |
| Group 2 | Group 1 | -4.100 | 0.000 |
| Group 3 | -3.500 | 0.000 |
| Group 3 | Group 1 | -0.600 | 0.577 |
| Group 2 | 3.500 | 0.000 |
| Day 10 | Group 1 | Group 2 | 3.400 | 0.000 |
| Group 3 | 0.600 | 0.563 |
| Group 2 | Group 1 | -3.400 | 0.000 |
| Group 3 | -2.800 | 0.000 |
| Group 3 | Group 1 | -0.600 | 0.563 |
| Group 2 | 2.800 | 0.000 |
Comparison (p-value) with group 3 for pain assessment by Games-Howell’s post-hoc test.
| Dependent Variable | Mean Difference | Sig. |
|---|
| Day 1 | Group 1 | 1.100 | 0.171 |
| Group 2 | -0.700 | 0.455 |
| Day 4 | Group 1 | 0.900 | 0.237 |
| Group 2 | -3.400 | 0.000 |
| Day 7 | Group 1 | 0.600 | 0.392 |
| Group 2 | -3.500 | 0.000 |
| Day 10 | Group 1 | 0.600 | 0.352 |
Discussion
OA is an age related degenerative disorder with 8-16% of the population having clinical evidence of TMJ involvement [1,4]. TMJ OA is more prevalent in female. It may be because of alpha polymorphism of estrogen receptor [1]. Similarly, there was increased female prevalence (56.7%) in this study, which was comparable to those published by Alexiou KE et al., [4]. The frequency for OA increases with increasing age [1]. Bromelain has potential to reduce neutrophil migration and pro- inflammatory cytokines secretion and thus have anti-inflammatory properties. Trypsin shows anti-oxidant effects and it affects the protease activated receptor 2 activation, which reduces the inflammatory response [9]. Rutoside trihydrate has the potential to inhibit pro-inflammatory genes transcription in human macrophages [10]. The dosage used for oral enzymes was rutoside trihydrate 100 mg, bromelain 90 mg, trypsin 48 mg, which is an acceptable dosage and was used as enteric coated tablets. In this study, patients between 40–60 years of age were included with an average age of 49 years. The average age was found to be similar with other studies [4]. The pain scoring in the trial was done based on numeric rating scale on each visit of the patient, because it is easily understandable and is more practical as compared to other scales such as VAS [8]. Gastrointestinal adverse effects were avoided using diclofenac 50 mg bid, because short-term use of this dose maintains the efficacy and limits complications [5]. No systemic adverse reactions were reported during treatment and after the cessation of drug intake. Based on the response of treatment in all the groups, oral enzymes preparation showed similar efficacy, tolerability and found to be equally effective as diclofenac. The results in this trial showed same result between group 1 and 3 as reported by other authors Akhtar NM et al., Tilwe GH et al., Singer F et al., mentioned in the literature [11-13]. However, this baseline study also gives scope of replacing diclofenac, which has well documented adverse effects as compared to newer formulation of oral enzymes preparation. This study demonstrated the significant difference in management of TMJ OA using oral enzymes and diclofenac combination therapy in comparison to diclofenac or oral enzymes preparation. This combination drug was showing early and marked reduction in pain and found to be superior in the management of TMJ OA. This was the first study using oral enzymes and diclofenac combination for treatment of OA involving the TMJ. The encouraging results should prompt clinical trials on more number of patients of TMJ OA to further evaluate the therapeutic usefulness and application of this drug, so that newer formulation can be used to treat the disease.
Limitation
There were few limitations of the study, such as severity of pain perception was subjective, pretreatment clinical scoring was showing variation within individuals and within groups and the study was of short duration with a short term follow-up.
Conclusion
Based on the findings of this trial, the use of this combination therapy is a promising new treatment modality to reduce pain in TMJ OA. Further studies and research are required to confirm the above results and to determine efficacy for long- term use of this combination drug.