Introduction
Amlodipine is a third generation dihydropyridine group of calcium channel blocker and having an excellent antihypertensive profile. Pedal Oedema (PE) is the major drawback of amlodipine therapy and the incidence of Amlodipine Induced Pedal Oedema (AIPE) has been found significantly high. Several neurohumoral factors influence the incidence of oedema.
Aim
We aimed to compare the plasma levels of renin, vasopressin and atrial natriuretic peptide in hypertensive AIPE, non-oedema and cilnidipine treated patients.
Materials and Methods
The present prospective, interventional study was conducted on 104 mild to moderate hypertensive patients (52 patients in each group), after due consideration of eligibility criteria. Plasma Renin (PR), Vasopressin (VAS), and the Atrial Natriuretic Peptide (ANP) was estimated by ELISA test and compared between the AIPE, Amlodipine Treated Non-Oedema (ATNE) in Phase I, and AIPE and Cilnidipine Treated (CT) Groups in Phase II.
Results
The clinical and demographic parameters were matched. PR was significantly high in AIPE group than the ATNE, and it was significantly reduced after one month follow up with the substitution of cilnidipine. The median (IQR) value of PR was 4.87 (3.58, 6.63), 3.50 (1.44, 5.47) and 2.66 (1.02, 5.66) ng/ml in AIPE, ATNE, CT group respectively.
VAS was significantly high in AIPE group than ATNE, and it significantly reduced after one month follow up with CT group. The median (IQR) value of vasopressin was 6.78 (2.55, 9.16), 2.58 (1.61, 5.73) and 2.50 (1.23, 5.00) ng/ml in AIPE, ATNE and CT groups respectively.
There was no significant difference seen in plasma ANP levels between the groups. The p-value was <0.05 which is statistically significant.
Conclusion
The AIPE may not be volume overload or fluid retention; it may be due to persistent raise in adrenergic activity followed chronic amlodipine therapy. Cilnidipine relatively suppresses the sympathetic activity, and completely resolves the AIPE by significantly reducing PR and VAS levels. ANP did not show a difference between groups. Cilnidipine is the suitable alternative antihypertensive drug for AIPE patients.
Introduction
Hypertension (HTN) is a major health concern all around the world. One billion people are affected worldwide; the prevalence of HTN increases as age advances. The risk factors for the development of HTN includes diet, lifestyle factors, particularly obesity and also genetic factors [1]. The persistent high Blood Pressure (BP) damages the chief organs like kidney, liver, eye, and brain. The early detection of HTN and better treatment require to lead a good quality of life [2-4].
The pharmacological modalities for HTN mangement include diuretics, Angiotensin-Converting Enzyme Inhibitors (ACEIs), Angiotensin Receptor Blockers (ARBs), Beta-Blockers (BBs), Calcium Channel Blockers (CCBs), vasodilators, which can be prescribed as a monotherapy or in combination. The choice of the antihypertensive drugs depends on the severity of the disease [5,6]. Initially, dihydropyridine (DHP) group of CCBs introduced in the 1960s for the treatment of angina [7]. DHP group of CCBs have undergone numerous alterations to improve their efficacy. Amlodipine is a third generation DHP-CCB with the good pharmacological profile [8]. Currently, Cilnidipine is available as a fourth generation DHP-CCB [9].
Cilnidipine is a novel and unique DHP anti-hypertensive drug. It acts on L and N-types of calcium channels. Cilnidipine decreases the BP by blocking L-type Ca2+ channels and relaxes the vascular tone, and it inhibits N-type Ca2+ channels, thus suppressing Sympathetic Activity (SA). In hypertensives, cilnidipine shows less sympathetic activity than amlodipine. The additional clinical advantage is that it helps to treat AIPE patients and completely resolves the oedema [10].
PE is the major drawback of amlodipine [10]. The incidence of AIPE has been found to be between 1.7% and 63.3% in different clinical studies [2,11]. Almost, the majority of the patients terminated the amlodipine therapy due to its adverse effect and switched to other class of antihypertensive drugs [2].
Accumulation of excess fluid in interstitial spaces leads to oedema. Several factors influence the formation of oedema, the major factors that influence the oedema are increased Hydrostatic Pressure (HP), decreased Colloidal Osmotic Pressure (COP), venous congestion, upright posture, pregnancy, obesity, heart failure, liver disease, kidney diseases [12]. The increased neurohumoral factors like secretion renin, Angiotensin-II, Aldosterone (ALDO), VAS and catecholamines lead to the formation of oedema [13].
The Renin-Angiotensin-Aldosterone System (RAAS) is involved in the body homoeostasis and blood pressure regulation. The enzyme renin releases from Juxtaglomerular (JG) cells into the circulation in response to volume overload. The complex mechanism involved in the release of renin, and also numerous systemic factors are involved, for example, salt intake, arterial blood pressure, and the sympathetic nervous system [14]. The ANP is a regulating hormone, which inhibits the release of renin, aldosterone, VAS and catecholamines [15]. Nitric Oxide (NO) inhibits the release of ANP [16]. The exact mechanism of ankle oedema by CCBs is not clearly understood. There are several studies reported the hypothetical mechanism and pathophysiology of AIPE [2,6,10,17]. However, there is no study conducted on neurohumoral factors in AIPE, ATNE and CT patients.
Hence, the present study was conducted to compare the involvement of the pr activity, VAS and ANP levels in hypertensive AIPE and ATNE and Cilnidipine Treated (CT) patients.
Materials and Methods
The present prospective, interventional study was carried out in the Department of Cardiology, Kasturba Medical College Hospital, Manipal University, Manipal, Karnataka,
Study duration: Three years starting from November 2013 to November 2016.
Ethics: Institutional Ethics Committee (IEC) approval took before study commences (Approval no. IEC 681/2013). The patient’s Information Sheet (PIS) was given to all patients and explained about the present study and written informed consent obtained before study from all participants after explaining the details and risks involved in the study.
Study plan: [Table/Fig-1]
Phase 1-Observational
Phase 2-Interventional
Describing the study plan.
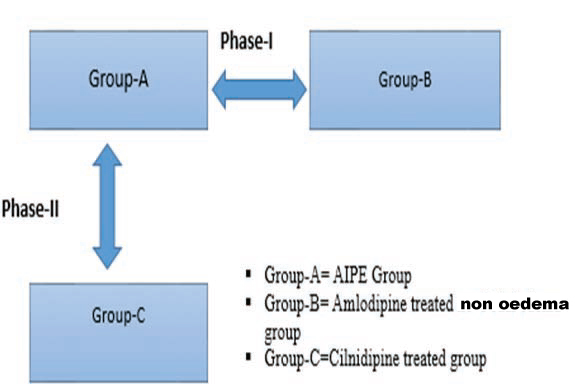
Phase-I study: Phase-I study was as observational study between AIPE group and ATNE group (52 n, in each group).
Phase-II study: Phase-II study was an interventional study, clinical and biochemical parameters (baseline) were noted in AIPE group; an equipotent dose of cilnidipine was substituted instead of amlodipine and followed up for one month, and reassessed the clinical and biochemical parameters (n=52).
Sample size calculations: The formula was used was “sample size for comparison for comparison of two means
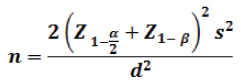
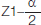 = 1.96 at 5% level of significance.
= 1.96 at 5% level of significance.
Z1-β = 1.28 for 90% power.
S2 = Pooled variance calculated from the pilot study. (3.5 x 3.5 =12.24)
d2 = Clinically significant difference. (1)
Considering a 95% confidence level and 90% of power, then the minimum required sample size was 42 in each group. In the present study, 52 subjects enrolled in each group.
Eligibility criteria
Inclusion Criteria: Mild to moderate hypertensive, AIPE patients of both genders {>140/90 mmHg}, Patients currently receiving amlodipine more than six months for the treatment of HTN, age range between 18 to 70 years, and patients should present with AIPE {with no other obvious cause}. In ATNE group, all the inclusion criteria were same as in the AIPE group, except they should not develop AIPE.
Exclusion criteria: Patients with major organs diseases like kidney, heart, liver and lung disease excluded. The endocrine abnormalities, peripheral vascular diseases, stroke, pregnant and lactating women, patients with any other class of antihypertensive medication were excluded. Exclusion criteria were same for all the groups.
Study procedure: Initially, the demographic parameters, clinical examinations, vitals values were noted. The oedema was confirmed by gently pressing thumb over the foot or ankle. The demographic, clinical and biochemical parameters (PR, VAS, ANP) between AIPE and ATNE group (Phase-I, Observational study) were compared. In the phase-II of study, same AIPE group took as a baseline, and an equipotent dose of cilnidipine was substituted instead of amlodipine, followed up for one month and clinical and biochemical parameters reassessed (n=52). (Phase-II study was an interventional study). Before collection of blood samples, the patient was advised to keep calm and relax on the bed for 30 minute in the supine position. A blood sample was collected in 2 ml of EDTA vacutainer tube (BD). The collected blood samples centrifuged immediately for 15 minutes at 5000 rpm at 4-8°C temperature. Immediately after centrifugation process separated plasma was aliquoted and stored a deep freezer at -70°C for further analysis. The collected samples were processed in ELISA kit method as instructed by the manufacturer. Repeated freeze-thaw cycles avoided.
ELISA Kits: RayBio® Human Renin ELISA Kit, RayBio® Human/ Mouse/Rat ANP ELISA Kit, RayBio® Human/Mouse/Rat Vasopressin ELISA Kit was used to estimating the PR, ANP and VAS levels respectively.
Statistical Analysis
Continuous variables were summarised as mean with Standard Deviation (SD) or median with interquartile range (IQR) as applicable. Variation in test parameters across the test groups was assessed through Kruskal-Wallis H test. All test of significance were two-tailed with a p-value <0.05, indicating statistical significance. Data analysis performed using Statistical Package for Social Sciences software, SPSS (Version 15.0).
Results
The present study was a prospective, interventional study on 104 patients; all patients completed the study. There was no significant difference in demographic parameters. The mean age of patients was 58.38±10.46 and 58.40±8.7 years, and weights were 61.54±9.11 and 64.42±9.3 kg; the mean height was 156.9±10.24 and 159.5±8.6 cm., BMI was 25.05±2.8 and 25.22±2.7 m2 in AIPE and ATNE groups respectively. Type 2 Diabetes Mellitus (DM) patients in AIPE group were 25 (48.07%), and ATNE group was 23 (44.23%). Demographic parameters and all other aspects compared and noted in [Table/Fig-2].
Comparison of demographic parameters of AIPE and ATNE.
| Variables | AIPE (n=52)(%) (mean ± SD) | ATNE (n=52)(%) (mean ± SD) | p-value* |
|---|
| Age (years) | 58.38 ±10.46 | 58.40 ± 8.7 | 0.992 |
| Weight (Kg) | 61.54 ±9.11 | 64.42 ±9.3 | 0.138 |
| Height (cm) | 156.9 ± 10.24 | 159.5 ± 8.6 | 0.139 |
| BMI (kg/m2) | 25.05 ± 2.8 | 25.22 ±2.7 | 0.758 |
| T2 DM | 25 (48.07%) | 23 (44.23%) | 0.556 |
| Gender (M/F) | 22/30 | 25/27 | 0.239 |
Variables were summarised as mean ± SD and percentage, comparison of variables between the groups using Chi-square test and independent t-test, a p-value <0.05 indicates statistical significance. AIPE: Amlodipine induced pedal oedema, ATNE: Amlodipine treated non- oedema. BMI: Body mass index, T2 DM: Type 2 Diabetic mellitus
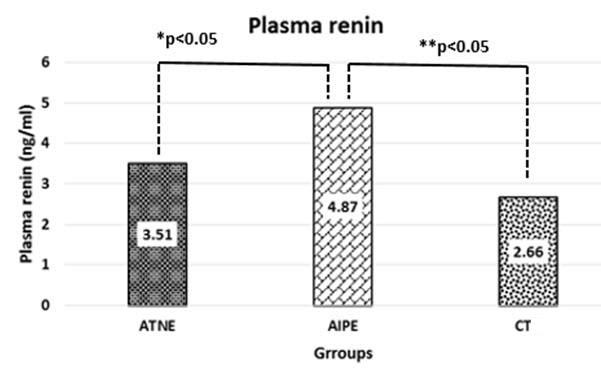
The median of PR was 4.87 (3.58, 6.63) and 3.50 (1.44, 5.47) ng/ml in AIPE and ATNE groups respectively, which is statistically significant (p<0.05).
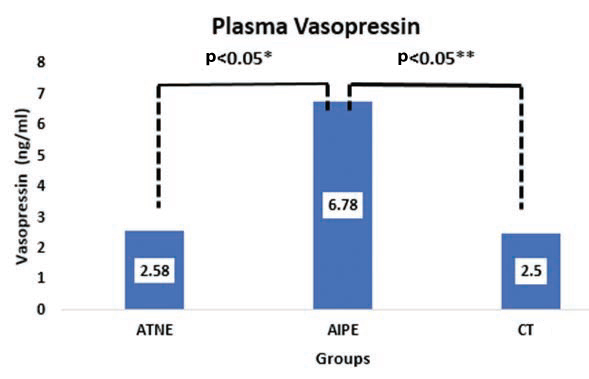
The median value of VAS was 6.78 (2.55, 9.16) and 2.58 (1.61, 5.73) ng/ml, which is which is statistically significant (p<0.05). The median value of ANP was 17.28 (7.21, 25.63) and 20.53 (11.18, 40.08) pg/ ml; there was no significant statistically difference, summarised in [Table/Fig-3].
Comparison of neurohumoral parameters between AIPE and ATNE groups.
| variables | AIPE (n = 52) median (IQR) | ATNE (n = 52) median (IQR) | p-value* |
|---|
| PR (ng/ml) | 4.87 (3.58,6.63) | 3.50 (1.44,5.47) | 0.007 |
| VAS (ng/ml) | 6.78 (2.55,9.16) | 2.58 (1.61,5.73) | 0.001 |
| ANP (pg/ml) | 17.28 (7.21,25.63) | 20.53 (11.18,40.08) | 0.158 |
*Comparison of plasma renin, vasopressin, atrial natriuretic peptide hormone between AIPE and ATNE groups (n=50 in each group). Continuous variables expressed in the median with IQR, by using Mann-Whitney U test.
p<0.05. is consider being statistically significant. Abbreviations: -AIPE: Amlodipine induced pedal oedema, ATNE: Amlodipine treated non oedema, PR: Plasma renin. VAS:Vasopressin, ANP: Atrial natriuretic peptide
The median of PR was 4.87 (3.58, 6.63) and 2.66 (1.02, 5.66) ng/ ml in AIPE and CTG respectively, which is statistically significant (p<0.05). The median value of VAS was 6.78 (2.55, 9.16) and 2.50 (1.23, 5.00) ng/ml, which is statistically significant (p <0.05). The median value of ANP was 17.28 (7.21, 25.63) and 17.50 (10.60, 25.63) pg/ml; there was no significant statistically difference, noted in [Table/Fig-4].
Comparison of neurohumoral parameters before and after substitution of cilnidipine treatment among AIPE group (n=52).
| variables | Before cilnidipine substitution median (IQR) | After cilnidipine substitution median (IQR) | p-value* |
|---|
| PR (ng/ml) | 4.87 (3.58,6.62) | 2.66 (1.02,5.66) | 0.001 |
| VAS (ng/ml) | 6.78 (2.55,9.16) | 2.50 (1.23,5.00) | <0.001 |
| ANP (pg/ml) | 17.28 (7.21,25.63) | 17.50 (10.60,25.63) | 0.461 |
Variables between the groups were compared using Wilcoxon signed rank test. Significant p- value i.e. <0.05 are shown in bold face PR: Plasma renin.VAS:Vasopressin, ANP: Atrial natriuretic peptide
The median of PR was 3.51 (1.44, 5.47) and 2.66 (1.02, 5.66) ng/ ml in ATNE and CT groups respectively, which is statistically not significant (p>0.05). The median value of VAR was 2.58 (1.61, 5.73) and 2.50 (1.23, 5.00) ng/ml, which is statistically not significant (p>0.05). The median value of ANP was 20.53 (11.18, 40.08) and 17.50 (10.60, 25.63) pg/ml; there was no significant statistically difference, noted [Table/Fig-5].
Comparison of neurohumoral parameters before and after substitution of cilnidipine treatment among AIPE group (n=52).
| Variables | ATNE (n = 52) median (IQR) | CT (n=52) median (IQR) | p-value* |
|---|
| PR (ng/ml) | 3.51 (1.44,5.47) | 2.66 (1.02,5.66) | 0.416 |
| VAS (ng/ml) | 2.58 (1.61,5.73) | 2.50 (1.23,5.00) | 0.142 |
| ANP (pg/ml) | 20.53 (11.18,40.08) | 17.50 (10.60,25.63) | 0.323 |
The comparison of plasma renin, vasopressin, atrial natriuretic peptide hormone between AIPE and ATNE groups (n=52 in each group). Continuous variables were expressed as median with interquartile range (IQR). Continuous variables compared by Mann-Whitney U test.
p<0.05. Is consider being statistically significant.n Abbreviations: -AIPE: Amlodipine induced pedal oedema, CT: Cilnidipine treated group, PR: Plasma renin. VAS:Vasopressin, ANP: Atrial natriuretic peptide
[Table/Fig-6] showing the comparison of PR between the three groups, where between ATNE and AIPE; AIPE and CT showed a significant difference (p<0.05). But there was not much difference in between ATNE and CT group.
Bar-diagram showing the comparison of plasma renin between AIPE and ATNE and CT.
[Table/Fig-7] showing the comparison of VAS between the three groups, where between ATNE and AIPE; AIPE and CT showed a significant difference (p<0.05). But there was no difference seen between ATNE and CT group.
Bar-diagram showing the comparison of vasopressin between AIPE, ATNE and CT.
Discussion
The RAAS is a principal system to maintain the blood pressure and body fluid homoeostasis, and renin controls this system. The multiple factors involved in the release of renin, such as sympathetic activity, salt intake, blood pressure, prostaglandins. The secretion of renin involves G-protein– coupled receptors activation, thereby activating adenylyl cyclase activity and cAMP generation [17-19]. In the present study, the PR and VAS were significantly high in AIPE group than the ATNE group. Cappuccio F et al., conducted a study on the effect of amlodipine on plasma renin activity and ANP they concluded that amlodipine increases the PR activity and a decrease in ANP levels [8]. The hypertension related stress and chronic therapy of amlodipine enhance the release of more catecholamine to circulation [20], which possibly stimulates the release of the renin from JG cells [21]. The renin initiates the cascade of reactions like; renin activates the angiotensin I to active angiotensin II through ACE, the active angiotensin II stimulates numerous of factors, like its release of VAS or ADH from the pituitary gland which acts on collecting duct and reabsorbs the water. It is a potent arteriolar vasoconstrictor and increases the BP. It stimulates the adrenal cortex to release the aldosterone which enhances the tubular reabsorption sodium chloride and water. Angiotensin II itself stimulates the SA [22]. In the present study, AIPE group showed higher VAS level than the other two groups. Cilnidipine is a fourth generation CCB, which acts on L/N type of calcium channel and effectively suppresses the neurohumoral regulations in the cardiovascular system and inhibits the release of catecholamine from the neuronal terminal by this action SA been reduced by cilnidipine treatment [9]. In the present study, AIPE group showed higher PR and VAS; the same group after one month with the substitution of cilnidipine showed significantly lesser PR and VAS level than baseline, this result gives evidence that CT group suppresses the neurohumoral activity, along with decreasing SA. Takahara A, concluded an animal study with decreased PR, norepinephrine, aldosterone after cilnidipine therapy [23]. Aritomi S et al., conducted a study on evaluation of neurohumoral activity by combined administration of valsartan and amlodipine or cilnidipine; they concluded that there is a decrease in PR activity, catecholamines, aldosterone, angiotensin II [23,24], these two studies animal studies supports our study results. ANP is a potent vasodilator, which is involved in the homeostatic control of body water and electrolytes. ANP is secrets from atrial muscles of the heart in response to volume overload, and it decreases the BP [25], and it precisely inverts the activity of aldosterone [26]. In the present study, we compared the ANP levels in three study groups; there was no statistically significant difference seen, but in ATNE group showed slightly higher ANP levels than the other groups which possibly inhibits the activation of RAAS. The cause of AIPE mechanism is still unclear, so the present study results suggest the possible mechanism, that the increased catecholamine release from the chronic amlodipine therapy and disease-related stress simulates the RAAS, which reabsorbs the more water and Na+ from collecting duct where cascade mechanism involved. Amlodipine acts on only L-type of calcium, it decreases the peripheral vascular resistance by relaxing the arteriolar smooth muscle and increases the hydrostatic pressure, infiltration will be more [6]. Cilnidipine is L/N types of CCB, and it dilates the arteriole by acting on L - type of Ca2+ channels at vascular smooth muscles and N type Ca2+ channels are present in the neuronal terminals and have a principal role in the regulation of SA [10]. Sympathetic nerve endings are spread over the venules and have constrictive action results in an imbalance in precapillaries and postcapillaries, by blocking N type calcium channels, decreases the release catecholamine from neuronal terminals [27], so the cilnidipine treatment causes venular dilation. This unique mechanism of action of cilnidipine results in vasodilation of both pre capillary and post capillary resistance vessels, and as well as brings down capillary hypertension and hyperfiltration of fluid into interspatial specs.
Limitation
The present study design was a prospective interventional follow up study. A single visit took into consideration in ATNE group; this might have led to some bias. We have processed stored samples (one month) it may affect the results.
Conclusion
The AIPE is may not be volume overload or fluid retention; it may be due to persistent raise in adrenergic activity followed chronic amlodipine therapy. Cilnidipine relatively suppresses the SA, and completely resolves the AIPE by significantly reducing PR and VAS levels. ANP did not show a difference between groups. Cilnidipine is the suitable alternative antihypertensive drug for AIPE patients.
*Variables were summarised as mean ± SD and percentage, comparison of variables between the groups using Chi-square test and independent t-test, a p-value <0.05 indicates statistical significance. AIPE: Amlodipine induced pedal oedema, ATNE: Amlodipine treated non- oedema. BMI: Body mass index, T2 DM: Type 2 Diabetic mellitus
*Comparison of plasma renin, vasopressin, atrial natriuretic peptide hormone between AIPE and ATNE groups (n=50 in each group). Continuous variables expressed in the median with IQR, by using Mann-Whitney U test.
*p<0.05. is consider being statistically significant. Abbreviations: -AIPE: Amlodipine induced pedal oedema, ATNE: Amlodipine treated non oedema, PR: Plasma renin. VAS:Vasopressin, ANP: Atrial natriuretic peptide
*Variables between the groups were compared using Wilcoxon signed rank test. Significant p- value i.e. <0.05 are shown in bold face PR: Plasma renin.VAS:Vasopressin, ANP: Atrial natriuretic peptide
The comparison of plasma renin, vasopressin, atrial natriuretic peptide hormone between AIPE and ATNE groups (n=52 in each group). Continuous variables were expressed as median with interquartile range (IQR). Continuous variables compared by Mann-Whitney U test.
*p<0.05. Is consider being statistically significant.n Abbreviations: -AIPE: Amlodipine induced pedal oedema, CT: Cilnidipine treated group, PR: Plasma renin. VAS:Vasopressin, ANP: Atrial natriuretic peptide
[1]. Soubra L, Nureddin H, Omar AG, Saleh M, Factors associated with hypertension prevalence and control among lebanese hypertensive type2 diabetic patientsInternational Journal of Pharmacy and Pharmaceutical Sciences 2016 8:153-59. [Google Scholar]
[2]. Adake P, Somashekar H, Rafeeq PM, Umar D, Basheer B, Baroudi K, Comparison of amlodipine with cilnidipine on antihypertensive efficacy and incidence of pedal oedema in mild to moderate hypertensive individuals:A prospective studyJournal of Advanced Pharmaceutical Technology &Research 2015 6:81-86. [Google Scholar]
[3]. Anchala R, Kannuri NK, Pant H, Khan H, Franco OH, Di Angelantonio E, Hypertension in India:a systematic review and meta-analysis of prevalence, awareness, and control of hypertensionJournal of Hypertension 2014 32:1170-77. [Google Scholar]
[4]. Lillie EO, O’Connor DT, Early phenotypic changes in hypertension a role for the autonomic nervous system and heredityHypertension 2006 47:331-33. [Google Scholar]
[5]. Hoshide S, Kario K, Ishikawa J, Eguchi K, Shimada K, Comparison of the effects of cilnidipine and amlodipine on ambulatory blood pressureHypertens Res 2005 28:1003-08. [Google Scholar]
[6]. Shetty KK, Shetty RK, Ganiga NCS, Reddy RP, Nayak V, Calcium channel blockers induced pedal oedema;mechanism and treatment options:ReviewInternational Journal of Sciences & Applied Research 2015 2:27-33. [Google Scholar]
[7]. Aouam K, Berdeaux A, Dihydropyridines from the first to the fourth generation:better effects and safetyTherapie 2002 58:333-39. [Google Scholar]
[8]. Cappuccio F, Markandu N, Sagnella G, Singer D, Buckley M, Miller M, Effects of amlodipine on urinary sodium excretion, renin-angiotensin-aldosterone system, atrial natriuretic peptide and blood pressure in essential hypertensionJournal of Human Hypertension 1991 5:115-19. [Google Scholar]
[9]. Chandra KS, Ramesh G, The fourth-generation Calcium channel blocker:CilnidipineIndian Heart Journal 2013 65:691-95. [Google Scholar]
[10]. Shetty R, Vivek G, Naha K, Tumkur A, Raj A, Bairy K, Excellent tolerance to cilnidipine in hypertensives with amlodipine-induced oedemaNorth American Journal of Medical Sciences 2013 5:47 [Google Scholar]
[11]. Patil P, Kothekar M, Development of safer molecules through chiralityIndian Journal of Medical Sciences 2006 60:427 [Google Scholar]
[12]. Xi G, Keep RF, Hoff JT, Pathophysiology of brain oedema formationNeurosurgery Clinics of North America 2002 13:371-83. [Google Scholar]
[13]. Grassi G, Assessment of sympathetic cardiovascular drive in human hypertension achievements and perspectivesHypertension 2009 54:690-97. [Google Scholar]
[14]. Schweda F, Kurtz A, Regulation of renin release by local and systemic factorsReviews of physiology, biochemistry and pharmacology 161 2009 Springer:1-44. [Google Scholar]
[15]. Ruskoaho H, Lang R, Toth M, Ganten D, Unger T, Release and regulation of atrial natriuretic peptide [ANP]European Heart Journal 1987 8:99-109. [Google Scholar]
[16]. Takizawa S, Matsushima K, Fujita H, Nanri K, Ogawa S, Shinohara Y, A selective N-type calcium channel antagonist reduces extracellular glutamate release and infarct volume in focal cerebral ischemiaJournal of Cerebral Blood Flow & Metabolism 1995 15:611-18. [Google Scholar]
[17]. Dibona GF, Kopp UC, Neural control of renal functionPhysiological Reviews 1997 77:75-198. [Google Scholar]
[18]. Jensen BL, Schmid C, Kurtz A, Prostaglandins stimulate renin secretion and renin mRNA in mouse renal juxtaglomerular cellsAmerican Journal of Physiology-Renal Physiology 1996 271:F659-F69. [Google Scholar]
[19]. Hautmann M, Friis UG, Desch M, Todorov V, Castrop H, Segerer F, Pituitary adenylate cyclase–activating polypeptide stimulates renin secretion via activation of PAC1 receptorsJournal of the American Society of Nephrology 2007 18:1150-56. [Google Scholar]
[20]. De Champlain J, Karas M, Nguyen P, Cartier P, Wistaff R, Toal CB, Different effects of nifedipine and amlodipine on circulating catecholamine levels in essential hypertensive patientsJournal of Hypertension 1998 16:1357-69. [Google Scholar]
[21]. Aldehni F, Tang T, Madsen K, Plattner M, Schreiber A, Friis UG, Stimulation of renin secretion by catecholamines is dependent on adenylyl cyclases AC5 and AC6Hypertension 2011 57:460-68. [Google Scholar]
[22]. Paul M, Mehr AP, Kreutz R, Physiology of local renin-angiotensin systemsPhysiological reviews 2006 86:747-803. [Google Scholar]
[23]. Takahara A, Dual L/N-Type Ca2+channel blocker:cilnidipine as a new type of antihypertensive drugAntihypertensive Drugs 2012 29:30-44. [Google Scholar]
[24]. Aritomi S, Niinuma T, Ogawa T, Konda T, Nitta K, (b) Effect of an N-type calcium antagonist on angiotensin II-renin feedbackAmerican Journal of Nephrology 2011 33:168-75. [Google Scholar]
[25]. Widmaier EP, Raff H, Strang KT, Vander’s human physiology 2006 Boston, Mass, USAMcGraw Hill [Google Scholar]
[26]. Goetz K, Physiology and pathophysiology of atrial peptidesAmerican Journal of Physiology-Endocrinology and Metabolism 1988 254:01-15. [Google Scholar]
[27]. Kojima S, Shida M, Yokoyama H, Comparison between cilnidipine and amlodipine besilate with respect to proteinuria in hypertensive patients with renal diseasesHypertension Research 2004 27(6):379-85. [Google Scholar]