The perspective of management of AOM has been changing continuously. Current research is focused towards the latest development in scientific understanding of AOM and its proper management. Improper treatment and untreated cases of AOM can lead to serious complications, especially in children under two years. AOM is one of the most frequently diagnosed diseases in children and most children with AOM are treated routinely with antimicrobial agents; although, a longstanding debate is yet to determine the necessity to use antimicrobial drugs in children with uncomplicated AOM. The key factor to successful treatment is the choice of specific antimicrobial agent. Gradual increase of antimicrobial resistance and costs of antimicrobial therapy have emphasized the need of judicious and rational use of antimicrobial drugs [1,2].
Suspected bacteria and their antibiotic susceptibility pattern guide the choice of antimicrobial agents for treatment of AOM. Common bacteria known to cause AOM in children are Streptococcus pneumoniae, Haemophilus influenzae and Moraxella catarrhalis [3]. However, there is relative paucity of data on middle ear pathogens as tympanocentesis is not routinely performed in children with AOM. High dose amoxicillin exceed the Minimum Inhibitory Concentration (MIC) of S. pneumoniae in middle ear fluid. A 4 to 6 days treatment with high dose amoxicillin and clavulanate potassium has been found effective to eradicate S. pneumoniae from middle ear [4,5]. On the other side, the data show that 58% to 82% of H. influenzae are susceptible to regular and high dose amoxicillin. Data also suggest that 100% of M. catarrhalis obtained from upper respiratory tract are susceptible to amoxicillin-clavulanate potassium [3-5]. Hoberman A et al., reported that 10 days treatment with amoxicillin-clavulanate potassium in children between 6 to 23 months of age with AOM showed resolution of signs and symptoms and decreased overall symptoms burden [6]. American association of paediatrics also recommends amoxicillin-clavulanate potassium as the first line drug in treatment of AOM [7].
Cefpodoxime is a wide spectrum oral third generation cephalosporin. It is active against aerobic Gram-positive and Gram-negative bacteria as well as anaerobic organisms. Guidelines of American associations of paediatrics suggests that ceftriaxone, cefprozil and cefuroxime axetil can be used as alternative options for treatment of PAOM in under two years as well as over two years of age [7]. Against this backdrop, the present study was conducted to compare the efficacy and safety of cefpodoxime with amoxicillin-clavulanate potassium in PAOM in children below two years.
Materials and Methods
The study was a prospective longitudinal interventional study conducted in the Department of Paediatrics of Malda Medical College. Data were received from 40 children from paediatrics outpatient department over a four month period between June 2016 and September 2016. Inclusion criteria were as follow: Children of either sex between 6 to 23 months, clinically diagnosed as AOM. Written consent from the parents of the children was obtained and data from each child was recorded in the case record form. Children with hypersensitivity to study drugs, hearing loss and toxic signs were excluded from the study. Children below six months of age were excluded from the study due to their different physiological state.
The study was designed to demonstrate equivalence between two treatment groups with respect to their efficacy and safety. Equivalence limit was set at 10%, True mean difference was considered zero, expected standard deviation was 10%, power was 90% and α=0.05. Thirteen subjects were required in each treatment group. Primer of biostatistics software (version 5.0) was used to calculate the sample size.
Grouping
Children fulfilling the selection criteria, were randomly divided into two treatment groups. Randomization was done by coin toss. Children in Group A received amoxicillin-clavulanate potassium 30 mg/kg/day (amoxicillin base) in two divided doses for 10 days. Children Group B received cefpodoxime 10 mg/kg/day in two divided doses for 10 days. Children showing worsening clinical signs or treatment failure were withdrawn prematurely from the study. Apart from the study drugs, no concomitant medication was administered to the children. Safety monitoring was performed continuously throughout the study. All Adverse Effects (AE) spontaneously reported by the parents or elicited by the treating paediatrician were recorded. The causality analysis was done as per the World Health Organization-Uppsala Monitoring Centre (WHO-UMC) criteria [8].
Efficacy Parameter
The efficacy parameter was number of children achieving “treatment success” in each treatment group. Treatment success was based on changes in the AOM-SOS scores [9,10] [Table/Fig-1] at day 10 visit. It was subdivided into two categories: (a) “clinical cure” if the AOM-SOS score was ≤1 at day 10 visit or (b) “clinical improvement” if the AOM-SOS score was between 2 and 4 on day 10. “Treatment failure” was declared in case of requiring a modification of antibiotic therapy, resulting in hospitalization on day 10 or earlier or failure to respond to study drugs.
| Symptoms/ Scores | Ear tugging | Crying | Irritability | Difficulty in sleeping | Diminished activity | Diminished appetite | Fever |
|---|
| Score 0 | none | none | none | none | none | none | none |
| Score 1 | little | little | little | little | little | little | little |
| Score 2 | A lot | A lot | A lot | A lot | A lot | A lot | A lot |
Study Visits
Each child was treated for 10 days. Children were evaluated clinically at baseline (day 0) and at subsequent follow-up visits on Days five and 10. AOM-SOS score was recorded at every visit. Other clinical parameters such as congestion of the tympanic membrane, discharge etc., were also evaluated at each visit and compared with the baseline to assess the clinical response and denote resolution, improvement, or progression to define success or failure.
There was no statistically significant difference in the baseline demographic profile and baseline AOM-SOS scores. Changes in AOM-SOS scores from baseline have been shown in [Table/Fig-3].
Changes in AOM-SOS score in Group A and B.
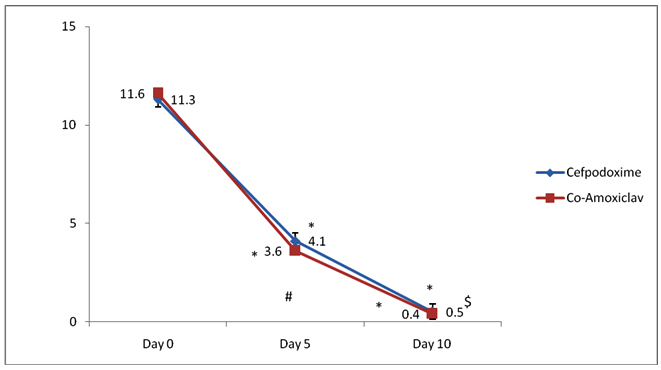
[Table/Fig-3] shows changes in AOM-SOS score in Group A and B. AOM-SOS score (mean± SD) in Group A are 11.6±0.5, 3.6 ±0.3, 0.4±0.1 at days 0, 5 and 10 respectively and in Group B are 11.3±0.6, 4.1±0.4, 0.5±0.2 at days 0, 5 and 10 respectively. Intra group comparison-* p<0.05−weeks 4, 8,12 versus week 0 in Group A and B. Statistical test used was Friedman’s test. Inter group comparison- # p=0.9-Group A versus Group B at day 5, p=0.3 Group A versus Group B at day 10. Statistical test used was Mann-Whitney U test.
Intra group analysis of AOM-SOS score at baseline (day 0) against day 5 and day 10 scores showed a highly significant decrease in both groups and clinically significant improvement in the signs and symptoms of the PAOM. Thus, it can be suggested that both cefpodoxime and amoxicillin-clavulanate potassium are effective in treatment of PAOM. Inter group analysis of the AOM-SOS scores showed that there was no statistically significant difference in the baseline, day 5 and day 10 AOM-SOS scores. So, it can be suggested that both cefpodoxime and amoxicillin-clavulanate potassium are equally effective in treatment of PAOM.
[Table/Fig-4] shows the percentage of children categorized as “treatment success” or “treatment failure” at day 10 visit. Fifteen children out of the 16 analysed in Group A achieved “treatment success,” i.e., either clinical improvement or clinical cure; remaining one was categorized as treatment failure. Similarly, in the Group B, 15 children out of the 17 evaluated showed “treatment success,” i.e., either clinical improvement or clinical cure, and the remaining two were categorized as treatment failure. Inter group comparison of the percentage of children who were categorized as treatment success showed no statistically significant difference (p=0.25). One child in Group A and two children in Group B were grouped as treatment failure. They were prescribed other antibiotics after day five evaluation.
Comparison of treatment success rates.
| Scores | Amoxicillin-clavulan- ate potassium group (n = 16) | Cefpodoxime group (n = 17) | p-value |
|---|
| Treatment success Clinical cure Clinical improvement | 15 (93.8%)141 | 15 (88.2%)141 | 0.25 |
| Treatment failure | 1 | 2 |
Statistical Analysis
Children who reported for at least one follow-up visit were analysed. All children were included for safety analysis. Friedman’s test was used for intra-group comparison. Mann-Whitney U test was used for inter group comparison. Chi-square test was used for categorical data. A p-value < 0.05 was considered to be statistically significant.
Results
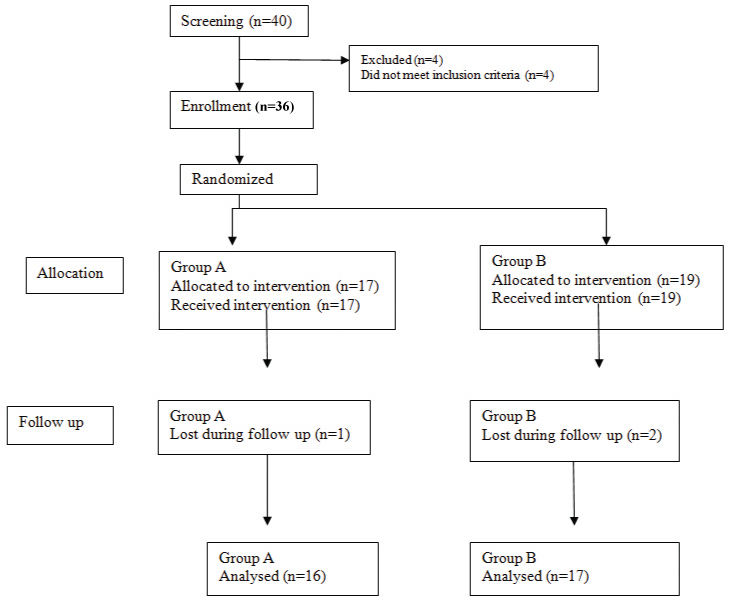
Out of 40 children screened, 36 fulfilled the selection criteria and included in the study. The four children, who were excluded from the study, were given adequate treatment accordingly. Children fulfilling the selection criteria were randomly divided into two treatment groups, 17 children were administered amoxicillin-clavulanate potassium and included in Group A. Remaining 19 children were administered cefpodoxime and included in Group B as mentioned in the flow chart [Table/Fig-2]. The mean age of children was 1.8 years and 1.6 years in the Group A and B respectively. Almost, 73.3% boys in Group A and 60% boys in Group B were there.
Two AEs were reported during the study period. Two boys in group A reported to have mild diarrhoea. None of the children in group B reported any AE. These were mild AEs and did not require modification of drug therapy. They were categorized as “possible” category. Over all safety profile of both the study drugs was good.
Discussion
Our study showed that cefpodoxime and amoxicillin-clavulanate potassium are equally effective in clinically diagnosed cases of PAOM, both in terms of efficacy and safety. After treatment with a 10-day course, the clinical success rates were comparable i.e., 88.2% in the cefpodoxime group and 93.8% in the amoxicillin-clavulanate potassium group. The incidence of AEs was also minimal i.e., two in the amoxicillin-clavulanate potassium group. These non-serious AEs did not require dose modification or withdrawal of drug therapy. Several published studies [6,11-14] show the efficacy of amoxicillin-clavulanate potassium for the treatment of PAOM. Few studies have also proved the efficacy of ceftriaxone, cefaclor and cefuroxime axetil in children with PAOM [7]. But no published data are available that compared amoxicillin-clavulanate potassium with an oral third-generation cephalosporin like cefpodoxime in PAOM.
The study conducted by Hoberman A et al., evaluated children between 6 to 23 months of age with AOM. They reported that treatment with amoxicillin-clavulanate potassium for 10 days resulted in quick recovery and decreased signs and symptoms of AOM on otoscopic examination [6]. The result proved that amoxicillin-clavulanate potassium is the first line drug for the treatment of PAOM in children below two years. Few studies have evaluated efficacy of oral cephalosporins in treatment of PAOM [14-17]. One multi-centric prospective clinical trial [14] compared the efficacy and safety of cefaclor and amoxicillin-clavulanate potassium in children with AOM. Both cefaclor and amoxicillin-clavulanate potassium caused a significant improvement in all the signs and symptoms after 10 days of treatment period. Inter group comparisons showed that the decrease in most of the symptoms was significantly higher in cefaclor arm as compared to amoxicillin-clavulanate potassium arm. The study showed cefaclor is well tolerated and effective treatment option for AOM in children and it is superior to the combination of amoxicillin-clavulanate potassium in efficacy and tolerability in AOM. However, the children included in the study by Agarwal M et al., were above two years of age. The mean age of the children in cefaclor group was 5.74 years and in amoxicillin-clavulanate potassium group was 4.93 years which is higher than our study group [15]. The guidelines of American Academy of Paediatrics for treatment of PAOM suggests amoxicillin, amoxicillin-clavulanate potassium, erythromycin, clarithromycin and cefuroxime as first line antibiotics drugs and clindamycin and ceftriaxone as second line antibiotic drugs [5]. Intravenous or intra-muscular administration of ceftriaxone is recommended in treatment of PAOM if amoxicillin is used within last one month for treatment of same condition [16]. But, there is no such guideline available in India and till date no study was conducted to compare cefpodoxime, an oral third generation cephalosporin in PAOM in children below two years. Our study shows that cefpodoxime and amoxicillin-clavulanate are equally effective in clinically diagnosed cases of PAOM, both in terms of effectiveness and safety in children below two years.
Some limitations of this study are as follows. A randomized double blind clinical trial could not be conducted due to lack of resources. Secondly, the sample sized of our study was relatively small. Thirdly, we did not perform tympanocentesis to obtain fluid from middle ear for bacteriological culture of the cases since very often paediatricians start antimicrobial therapy empirically and more importantly to avoid any inadvertent injury to a child below two years. We conducted this study to provide information to paediatricians on the comparative efficacy of these two antibiotics as initial antibiotics for children with PAOM below two years of age based on clinical assessment scores.
Conclusion
Our study shows that efficacy and safety of 10 days therapy with cefpodoxime is comparable to that of amoxicillin-clavulanate potassium in PAOM in children below two years. Future trials are required to assess relapse rates and bacteriological cure to provide more scientific insight into the study.