In dental practice, majority of emergency appointments are due to traumatic dental injuries, and the main task for a dental clinician is to ensure the viability of the traumatised teeth [1,2]. According to the World Health Organization (WHO) classification for traumatised teeth, avulsion, or exarticulation is the complete displacement of a tooth from its alveolar socket due to traumatic injury [3,4].
Preservation of vitality of PDL cells attached to the root surface is of utmost importance in cases of avulsion injuries as this may aid in re-establishing the PDL favourably [5,6].
The American Association of Endodontists (AAE) recommends the use of HBSS as the storage medium of choice for treatment of avulsed teeth [11]. HBSS plays an important role in biomedical research having an advantage of promoting cellular growth [12]. This solution is commercially available and has a pH of 7.2 with an osmolarity of 320 mOsm/kg [13] making it highly biocompatible.
However, the major disadvantage of HBSS is that it is not easily available in most places where these traumatic events usually occur such as in school, home, camps, and sports field settings where people are physically active [11] and the cost is also a major prohibitive factor [14]. Coconut water is the nature’s most refreshing and nutritious drink with health benefiting properties. It is one of the highest sources of electrolyte known to man [15]. Coconut water has a high osmolarity (372 mOsm/l) because of the sugars present, which are primarily glucose and fructose [16].
Aloe vera is a popular plant for alternative medicine. Aloe vera gel is a transparent mucilaginous jelly contained in the parenchymatous cell of the plant [17]. It contains 99% water and over 75 nutrients. It has several beneficial pharmacological actions, like it is anti-inflammatory, antibacterial, antioxidant etc., [18]. The osmolarity of aloe vera ranges from 280-300 mOsm/l [17,19]. The purpose of this study was to evaluate the efficacy of storage medium which are easily available in India and are economical, like coconut water and aloe vera, in comparison to the other traditional storage media like HBSS in maintaining the viability of PDL cells of an avulsed tooth.
Materials and Methods
This in vitro study was carried out at Department of Pedodontics and Preventive Dentistry using infrastructural support from Department of Pathology and Microbiology, Saraswati Dental College, Lucknow from January to June, 2016. Approval for conduction of this in vitro study was taken by Institutional Research and Development Committee.
A total of 58 freshly extracted non carious mature human premolar teeth with closed apices undergoing extraction for orthodontic therapeutic purpose were obtained for this study. Sample size was determined using convenient sampling design. Earlier, number of studies have been conducted using similar sampling criteria [5,9,10,15,18,20,21]. Exclusion criteria included extracted teeth of patients with moderate to severe periodontal disease and those with advanced caries. All extractions were performed atraumatically. After extractions the teeth were held from the coronal region using forceps and 3 mm of PDL was scraped from the coronal portion with the help of a curette in order to remove any damaged cells. The samples were then divided randomly into one of the four experimental groups: Group I-HBSS, Group II-Coconut water, Group III-Aloe vera and Group IV-Saline which contained 12 samples per group. The positive control (Group V) and the negative control (Group VI) consisted of five samples each.
Freshly open coconut water and fresh extract from the leaf of aloe vera was used for each sample. Lower one inch of the aloe vera leaf base, the tapering point of the leaf top and the short, sharp spines located along the leaf margins were removed with Bard Parker (BP) blade no. 15. With the help of the same blade inner gelatinous part was extracted and collected in a petri dish.
Following extractions the samples were dried for 30 minutes (in this duration the scrapping of coronal PDL cells was carried out). This was followed by immersion of the samples in the experimental storage solution, according to the respective groups, for duration of 45 minutes. The positive control teeth were neither dried nor were they stored in any solution, but rather they were immediately treated with Collagenase Type II and Dispase for 30 minutes. The negative control teeth were bench dried for eight hours, with no follow up storage solution time, and then placed in the Collagenase Type II and Dispase. Each experimental tooth, after drying and soaking, was incubated for 30 minutes in 15 ml Falcon tubes (Tarsons, India) with a 2.5 ml solution of 0.2 mg/ml of Collagenase Type II (HiMedia Laboratories Pvt, Ltd., Nashik, India) and a 2.4 mg/ml solution of Dispase (HiMedia Laboratories Pvt, Ltd., Nashik, India) in phosphate-buffered saline (Sigma Aldrich, USA). After incubation, 50 ul of Fetal Bovine Serum (Invitrogen, India) was added to each tube. All tubes were then centrifuged for four minutes at 1000 r.p.m. Sterile micropipettes were used to remove the supernatant and labelling of the cells were done with 0.4% Trypan Blue (HiMedia Laboratories Pvt, Ltd., Nashik, India) for determination of viability, according to Polverini PJ and Leibovich SJ [22]. Light microscope with a haemocytometer was used to count the number of viable and non viable cells at 20X magnification.
The formula used to calculate viable cell percentage was:
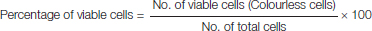
Statistical Analysis
The statistical analysis was done using SPSS (Statistical Package for Social Sciences) Version 15.0. The Analysis of Variance (ANOVA) and Post-hoc tests (Tukey-HSD) were performed to reveal the statistical significance. The values were represented in number (%) and mean±SD. The level of significance was considered at p-value 0.05 (at 95% confidence interval).
Results
In the present study a total of 58 samples were included and randomly allocated to one of the following groups. Cell viability (%) in different solution ranged from 2.52 to 99.22% respectively. Mean percentage of viable cells ranged from 3.75±1.22% (Group VI) to 98.64±0.54% (Group V). Amidst these extremes, the mean percentage of cell viability was 50.56±4.58% in Group IV, 70.59±4.73% in Group III, 79.87±3.86% in Group II and 87.33±5.24% in Group I. It was observed that the 95% confidence interval for mean of all the groups did not show any overlapping [Table/Fig-1].
Comparison of percentage of viable cells in each group.
| Group | N | Mean (%) | SD | SE | 95% Confidence Interval for Mean | Min | Max |
|---|
| Lower Bound | Upper Bound |
|---|
| I | 12 | 87.33 | 5.24 | 1.51 | 84.00 | 90.66 | 77.07 | 95.59 |
| II | 12 | 79.87 | 3.86 | 1.11 | 77.42 | 82.32 | 74.71 | 86.27 |
| III | 12 | 70.59 | 4.73 | 1.37 | 67.59 | 73.60 | 64.46 | 80.03 |
| IV | 12 | 50.56 | 4.58 | 1.32 | 47.64 | 53.47 | 44.20 | 57.01 |
| V | 5 | 98.64 | 0.54 | 0.24 | 97.97 | 99.32 | 98.00 | 99.22 |
| VI | 5 | 3.75 | 1.22 | 0.55 | 2.23 | 5.27 | 2.52 | 5.56 |
| Total | 58 | 68.49 | 25.19 | 3.31 | 61.86 | 75.11 | 2.52 | 99.22 |
(Group I- HBSS, Group II- Coconut water, Group III- Aloe vera, Group IV- Saline, Group V- Positive I control, Group VI- Negative control).
Between groups comparison revealed minimum difference between Groups I and II (7.46±1.75%) and maximum between Groups V and VI (94.90±2.70%). Between all the groups, differences were significant statistically (p≤0.001) [Table/Fig-2].
Between group comparison of number of total cells (Tukey HSD test).
| SN | Comparison | Mean difference | SE | ’p’ |
|---|
| 1. | I vs II | 7.46 | 1.75 | 0.001 |
| 2. | I vs III | 16.74 | 1.75 | <0.001 |
| 3. | I vs IV | 36.78 | 1.75 | <0.001 |
| 4. | I vs V | -11.31 | 2.28 | <0.001 |
| 5. | I vs VI | 83.58 | 2.28 | <0.001 |
| 6. | II vs III | 9.28 | 1.75 | <0.001 |
| 7. | II vs IV | 29.31 | 1.75 | <0.001 |
| 8. | II vs V | -18.77 | 2.28 | <0.001 |
| 9. | II vs VI | 76.12 | 2.28 | <0.001 |
| 10. | III vs IV | 20.04 | 1.75 | <0.001 |
| 11. | III vs V | -28.05 | 2.28 | <0.001 |
| 12. | III vs VI | 66.84 | 2.28 | <0.001 |
| 13. | IV vs V | -48.09 | 2.28 | <0.001 |
| 14. | IV vs VI | 46.81 | 2.28 | <0.001 |
| 15. | V vs VI | 94.90 | 2.70 | <0.001 |
On the basis of above evaluation, the following order of viable cells were observed in different experimental groups:
Positive control >HBSS >Coconut water >Aloe vera >Saline >Negative control
Discussion
Factors that might cause cellular damage to the PDL include extended extraoral period, dessication of PDL cells and unsuitable storage media [15]. Therefore, the basic strategy to be followed according to Andreasen JO and Hiorting-Hansen E, is immediate reimplantation or reimplantation as soon as possible following avulsion. According to him, early reimplantation (within 30 minutes) was more successful than the reimplantation carried out after longer periods of extraoral time [23]. But sometimes due to certain reasons immediate reimplantation is not possible. In those circumstances the tooth should be placed in a storage solution that preserve the PDL cell viability [5,6].
In the current investigation, the duration of drying was chosen as 30 minutes as this time period simulates a typical scenario wherein the avulsed tooth may remain dry before being placed into a storage medium.
In terms of storage time periods, earlier studies have used time frames of 30 minutes [18] or 45 minutes [5,9,10,15,21,24,25]. In the present study the teeth in the experimental storage solution were stored for 45 minutes. This time period was chosen as it allows for comparison with previous investigations.
To quantitate the number of viable PDL cells in the current study and to preserve maximum cell viability, the root surfaces were treated with Collagenase Type II and Dispase for rapid cell retrieval and cellular integrity [18]. Also, Fetal Bovine Serum was used as a growth supplement for cell culture media because of its high content of embryonic growth promoting factors [26]. The Trypan Blue staining technique was employed as it has advantages such as less time consumption, ease of operation and effective differentiation between non viable and viable cells [27].
HBSS is considered as a gold standard among all storage medium thereby making it an ideal reference medium for comparision with other media [28]. According to Hiltz J and Trop M, HBSS possess excellent properties for the maintenance of vital PDL cells. They also concluded that the cells appeared normal after storage in HBSS and 70% viable fibroblasts were maintained after duration of 96 hours in HBSS [29].
The health benefits provided by coconut water are well known to man since ancient time. According to Blomlof L, the osmolarity of a storage media is highly significant for the preservation of cell viability [30]. Coconut water having high osmolarity is extremely suitable for this purpose. It is also rich in many essential amino acids [16]. Mantena SK et al., has shown that coconut water possess antioxidant property due to the presence of ascorbic acid [31]. Buttke TM and Trope M, concluded that the viability of PDL cells was more effectively maintained in storage media with antioxidant as compared to the one without antioxidant property [32].
In the field of dentistry, aloe vera has been used to enhance defence mechanisms and hasten the healing of periodontal diseases by slowing or inhibiting the synthesis of thromboxane [25].
Saline solution contains 0.90% w/v of NaCl and has osmolality of 280 mOsm/kg. It is highly biocompatible, however it lacks nutrients such as magnesium, calcium and glucose [33] and due to this its use as a storage media is limited with a recommendation of storage for up to four hours [34,35].
In the present study HBSS, coconut water, aloe vera and saline were used as storage media and were compared. The result of the present study showed that the maximum percentage of viable PDL cells were found in the positive control group (98.64%) and the minimum percentage of viable cells were found in the negative control group (3.75%). Among experimental groups maximum percentage of viable cells were seen in HBSS (87.33%) followed by coconut water (79.87%), aloe vera (70.59%) and saline (50.56%) [Table/Fig-1].
Similar results were seen in study done by Omar SL et al. who found that the teeth stored in HBSS had highest percentage of viable PDL cells followed by tender coconut water and saline [15]. Thomas T et al., observed that HBSS had significantly higher number of viable cells as compared to that of coconut water [6]. Similarly, Fulzele P et al., found highest percentage of viable cells with HBSS followed by aloe vera and packaged drinking water [25]. Martin MP and Pillegi R; and Caglar E et al., in their studies found that HBSS demonstrated highest number of viable cells than saline [9,21]. These results acknowledge the previous studies by Ashkenazi M et al., which stated that HBSS was the most effective medium for preserving viability of PDL cells [36]. However, in contrast to the findings of present study, Gopikrishna V et al., found that coconut water kept significantly more viable PDL cells as compared to HBSS [5,10]. They concluded that the coconut water has higher osmolarity and this might have enabled the superior maintenance of cell viability.
Excellent performance of HBSS in maintaining the viability of PDL cells as shown in [Table/Fig-1] could be explained by the fact that HBSS is a pH balanced salt solution in which bacterial content is nil and has physiological osmolarity. It contains essential nutrients such as glucose, calcium, and magnesium ions, which can sustain and reconstitute the depleted cellular components of the PDL cells [2].
In the present study coconut water showed higher percentage of viable cells compared to aloe vera as shown in [Table/Fig-1]. The possible explanation for the better result of coconut water could be the presence of essential nutrients including proteins, amino acids, vitamins, and minerals which helps in nourishing cells thereby maintaining their viability. The primary sugars present in coconut water are glucose and fructose, which are responsible for the high osmolarity of coconut water that enables the superior maintenance of cell viability [10,16]. It has high potassium content and contains antioxidants attributed to ascorbic acid which is linked to a variety of health benefits. One of the most important constituent present in coconut water is cytokinins [20].
In spite of aloe vera having good medicinal properties, physiological pH, osmolarity and presence of an antioxidant enzyme, catalase [25], the present study showed that it maintained less percentage of viable cells as compared to coconut water [Table/Fig-1]. The reason which may reduce its effectiveness is that the pure aloe vera (at 100% concentration) is highly viscous and at the time of experiment it entirely covers the surface of the experimented cells which may have possibly prevented the accessibility of oxygen to them. As a result, more the deficiency in oxygenation, more the cell death will occur [37]. Badakhsh S et al., in their study found that the aloe vera at 10, 30 and 50% concentration preserved more PDL cells as compared to the aloe vera at 100% concentration [37].
Saline in the present study showed the least percentage of viable PDL cells among the experimental medium [Table/Fig-1]. The reason may be the lack of nutrients and essential metabolites necessary for maintaining cell viability [33].
Although in the present and other previous studies HBSS showed excellent potential in maintaining the highest percentage of viable PDL cells, it is not widely used in India because of its limited availability and high cost whereas in India, coconuts are more easily available as compared to the HBSS.
On the basis of result of the present study and beneficial properties of coconut water and aloe vera, both the medium have the potential to maintain the PDL cell viability of an avulsed tooth. Hence, both can be used as an alternative storage medium and are readily available in India even in the remotest place.
Limitation
A limitation of this in vitro study is that it does not mimic a real life scenario. Most avulsion injuries are likely to occur due to accidents, which could be related to sports, playground or road traffic injuries. In such cases the avulsed tooth might come in contact with surfaces such as the floor, ground, soil or road surfaces, which may result in bacterial contamination, thereby adversely affecting the viability of PDL cells. Furthermore, the extraoral dry time taken in this study was 30 minutes, whereas, in clinical scenario, there may be increased variations in extraoral dry time leading to adverse effects on PDL cells. Future research may focus on larger sample sizes for better corroboration of results. Easy accessibility of storage media like coconut water and aloe vera, especially in India, warrants further research regarding their efficiency in maintaining the viability of PDL cells.
Conclusion
Within the limitation of this study it can be concluded that among all the four experimental groups HBSS is the most effective storage media for an avulsed tooth. Coconut water proves to be a better storage media than aloe vera. The potential of storage medium for maintaining the PDL cell viability can be summarized as follows: HBSS >Coconut water >Aloe vera >Saline. Coconut water and aloe vera can be used as an alternative storage media for an avulsed tooth in areas where HBSS might be inaccessible and expensive.
(Group I- HBSS, Group II- Coconut water, Group III- Aloe vera, Group IV- Saline, Group V- Positive I control, Group VI- Negative control).