The term stress was borrowed from Physics by the Hans Selye who is been regarded as the “Father of Stress Research”. Stress is anything that disturbs the “homeostasis” of the body, the consequences of it been linked with increase in smoking, substance use, accidents and sleep related problems. Populations that live in more stressful environments (communities with higher divorce rates, business failures, natural disasters, etc.,) smoke more heavily and experience higher mortality from lung cancer and chronic obstructive pulmonary disorder [1]. It can also be defined as the inability of an animal to cope up with its surrounding environment [2]. Stress is characterized by physiological changes that occur in response to a new or a negative response [3]. Antidepressants are recommended for stress related disorders like depression [4]. Fluoxetine is a selective serotonin reuptake inhibitor group of antidepressant used in the treatment of wide range of mood disorders [5]. These drugs are also used in the management of stress, stress related depression and anxiety [6]. It has been used clinically as it is safe, few side effects and greater tolerability [7]. Since its early discovery, it was believed to inhibit the serotonin uptake which contributed to its therapeutic use. However, in the recent years, it has been suggested that its antioxidant property and neurogenic property might be the reason behind its therapeutic effect. This fact is still under cloud and is a topic of debate. The present study was done to evaluate the effect of fluoxetine in the hippocampus of cold restraint stressed male wistar albino rats.
Materials and Methods
Animals
The study was conducted in Biomedical Research Unit and Lab Animal Center (BRULAC), Saveetha University, Chennai, India (from January 2015 to February 2015). A total of 18 male wistar albino rats of weight around 150 g-200 g (12-16 weeks) were used (n=6). Prior ethical approval was obtained from the Institutional Animal Ethical Committee (SU/SMC/RD/001/2014). All the experimental procedures were carried out according to the Committee for the Purpose of Control and Supervision of Experiment on Animals (CPCSEA) guidelines. All the rats (three rats per cage) were housed in polypropylene cage under standard laboratory conditions with food and water provided ad libitum.
Experimental Design
The animals were divided randomly into the following groups.
Group 1- Control (given normal saline orally)
Group 2 – Cold restraint stress for 28 days (four weeks) –Negative control
Group 3 – Experimental group- The animals were treated with 10 mg/kg of the fluoxetine in combination with cold restraint stress.
The experimental groups were pretreated with fluoxetine for one week before the start of the stress paradigm [Table/Fig-1].
The experimental design for the study.
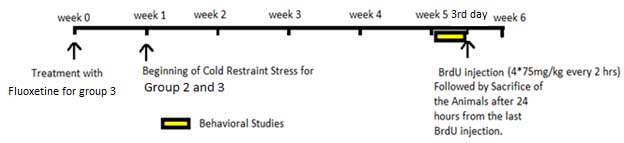
Drug Procuring and Dosage
The drug fluoxetine hydrochloride (Loftil) was purchased from a pharmacy in Chennai through proper channel. The drug was mixed along with water (vehicle) and was given to the animals orally by oral gavage technique. The animals in Group 3 were given a single dose of 10 mg/kg for a period of five weeks (one week pre-treatment+ four week treatment).
Stress Protocol
The animals of Group 2 and Group 3 were given cold restraint stress. The animals were immobilised at 4°C-6°C for 30 minutes for period of four weeks. The animals were then placed in their cages for a 5 minute re-adaptative period before receiving fluoxetine hydrochloride 10 mg/kg dissolved in normal saline (Group 3). Group 1 was the control group which was given only Normal saline and Group 2 was the stressed group which received only normal saline after cold restraint stress.
Blood Collection
Collection tubes containing sodium citrate as anticoagulant were used to collect the sample from the retro-orbital venous plexus with isoflurane inhalation. This procedure was done under the guidance of veterinary surgeon. Samples were centrifuged at 4°C for 15 minute at 3000 rpm. Small portion of it were aspirated and stored in sealable polypropylene microcentrifuge tubes at –20° C until assayed for serum corticosterone.
Corticosterone Levels
Corticosterone hormone is a stress marker used for assessing stress levels. High-Performance Liquid Chromatography (HPLC) was performed for serum corticosterone hormone estimation using a Shimadzu UHPLC (Japan) equipped with an autoinjector and an autosampler. Separations of the steroids were performed on a Hypersil Thermo C18 column (3.0 mm, i.d., x 50 mm, l.3 μm particle size). The injection volume was 5 μL and the oven temperature was 25°C. The mobile phase consisted of 5 mM ammonium acetate and methanol (25: 75, v/v) and was delivered at a flow rate of 0.25 mL/ minute. with PDA detector@ 242 nm.
Behavioural Studies
Before the sacrifice, Morris water maze test was conducted for all three groups at the end of the fifth week. The maze consisted of a black pool (6 feet in diameter, 3 feet high) filled with water and a black platform (10 cm in diameter). The escape platform was kept above the water surface by 1 foot during the initial training period and placed in the centre. During the testing period, water was further filled so that, the platform is hidden and the maze water was added with few drops of non fat milk to make the water appear opaque. The four starting locations were labelled North (N), East (E), South (S), and West (W) at an equal distance from the rim. The water was maintained at a temperature of 22±2°C. The temperature was checked and the pool was cleaned periodically.
On the first day after the end of treatment paradigm, all the animals of each group were given training three times by guiding them to reach the platform. On the second day, the rats were tested using a standard protocol. The time taken by each animal to reach the hidden platform was measured using a timer and the data was recorded manually into MS Excel 2016 for statistical analysis [8].
Sacrifice
The animals were sacrificed, 24 hours after the last injection of BrdU (Cell proliferative marker). The rats were deeply anaesthetized and was perfused transcardially with cold 0.1 M Phosphate Buffered Saline (PBS) followed by 4% paraformaldehyde after which the brain were dissected out. Later the brains were placed in 4% buffered formalin for 24-48 hours until the brain was properly fixed. Mid sagittal sections were made so that, one half of the brain was utilised for neurotransmitter and antioxidant level estimation and the other half was utilised for histological and immunohistochemical studies.
Estimation of Oxidative Stress Markers
Immediately after dissection of brain, homogenates were prepared from one half of the brain 10% tissues (w/v) in 0.05 M phosphate buffer, pH 7.4 or EDTA 0.02 M for the enzymatic assays.
Superoxide dismutase (SOD) was assayed according to the technique of Kakkar P et al., [9]. This method is based on the ability to inhibit the auto oxidation of pyrogallol at alkaline pH (8.2) by SOD. Catalase (CAT) activity was assayed by the method of Sinha AK [10]. The CAT activity was measured by rate of decomposition of hydrogen peroxide. Reduced glutathione (GSH) was assayed by following the method of Oliveria AC et al., [11]. GSH is measured by its reaction with 5,5’-dithionitro benzoic acid (DTNB) (Ellman’s reaction). Glutathione peroxidase (GPx) activity was determined by Hafeman DG et al., [12]. The activity was determined by measuring the decrease in the GSH content by incubating the sample in presence of hydrogen peroxide and sodium nitrite.
Analysis of Neurotransmitters Levels
Extraction medium: Brain samples were then homogenized in extraction medium. Extraction medium was prepared by 0.4 M perchloric acid with 0.1% sodium metabisulphite, 0.1% EDTA and 0.01% cysteine. Samples were then centrifuged at 10000 × g in Eppendorf tubes for 15 minutes at 4°C. Supernatants were used for HPLC analysis using fluorescent detector.
Estimation of monoamines and their metabolites: The endogenous levels of Nor Epinephrine (NE), Dopamine (DA), Serotonin (5HT) were determined by reverse phase HPLC with fluorescent detection. The separation was carried out on the reversed-phase Hypersil BDS-C18 column by a gradient elution. Eluent A was 30% of acetonitrile consisting of 30 mM ammonium formate buffer (pH 3.7); B was 100% of acetonitrile. The gradient elution program was as follows: 0 minute = 15% B, 5 minute = 15% B, 15 minute = 25% B, 25 minute = 100% B and 30 minute = 100% B. Before injecting the next sample, the column was equilibrated with the initial mobile phase for 10 min. The flow rate was constant at 1.0 mL/min and the column temperature was set at 30°C. The fluorescence excitation and emission wavelengths were set at λ maximum 333 nm and λ maximum 390 nm, respectively. The derivatives were quantified by fluorescence detector and identified simultaneously through HPLC retention time of standards and online ESI/MS structure identification.
Histological studies: After sacrifice, the tissues were processed for paraffin embedding and were stained for standard H&E. Darkly stained, shrunken cells and cells with fragmented nuclei were excluded from counting. Round, medium or large and clear cells with distinct nucleus were counted. Only adequately impregnated CA-3 pyramidal neurons emerging from the mouth of the dentate gyrus were selected for study.
BrdU immunohistochemistry: The fixed brain was processed immediately for immunohistochemistry for detection of Bromode-oxyuridine (BrdU) which is a thiamine analogue for localising rapidly dividing cells. For analysis of BrdU-positive cells, rats were administered BrdU intraperitoneally (75 mg/kg* four times in a gap of two hours between each) three days after the last treatment with fluoxetine. After sacrifice tissue blocks were prepared for paraffin embedding and sections were made of 15 microns. The standard staining procedure for immunohistochemistry for BrdU labelling for paraffin sections were followed and the peroxidase reaction was visualized by using 3,3’-diaminobenzidine tetrahydrochloride (DAB) as chromogen. Sections were counterstained with haematoxylin.
Cell counting: BrdU positive cells were counted in Subgranular Zone (SGZ) regions. Every 6th section was taken for quantitative assessment to ensure no BrdU positive cells are counted twice [13]. An average of seven sections in each tissue block were counted manually and the average was taken. The total number of BrdU positive cells per section was multiplied by six to include the total volume of the dentate gyrus region of the hippocampus [14]. Same microscope with same magnification was used. The cell region for calculation was selected using random selection technique from the serial sections made for each group. The normal pyramidal neurons in the CA3 region was counted using ocular micrometry.
Statistical Analysis
All the values are expressed as Mean±SEM (n=6). The data were tested for normality using Kolmogorov- Smirnov test with Dallal-Wilkinson – Lillie. One-way analysis of variance (ANOVA) followed by Tukey’s multiple comparisons test was used for the comparison of means. A probability of 0.05 and less was taken as statistically significant. The analysis and plotting of graphs were carried out using Prism Graph pad software version 6.01 (GraphPad Software, Inc USA).
Results
Results for Morris Water Maze Test
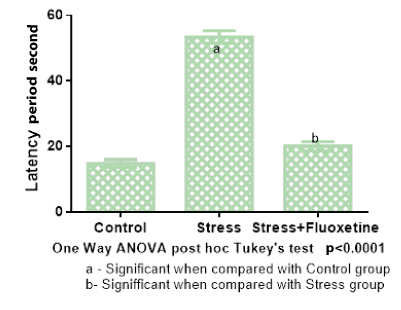
The mean and standard error for latency period in Group 1 (Control) was 14.8± 1.1, in Group 2 (cold restraint stress) was 53.5±1.8, in Group 3 (cold restraint stress with fluoxetine 10 mg/kg) was 20.2±1.4. One-way ANOVA test was performed for the latency period between the groups which showed that there was a statistically significant difference between groups (p<0.001) [Table/Fig-2].
Bar diagram showing the mean and SEM of latency period of rats (n=6).
Results for Oxidative Stress Markers
The mean and standard error for CAT, SOD, GSH and GPx levels were calculated and tabulated [Table/Fig-3].
The levels of antioxidants in brain.
| Group | CAT (μmol/min/ mg protein) | SOD (U/mg) | GSH (μmol /min/ mg of protein) | GPx (μmol /min/ mg of protein) |
|---|
| 1 | 25.55 ± 1.03 | 23.7±0.46 | 21.80±1.19 | 11.4±0.65 |
| 2 | 9.89 ±0.5 | 17.03±2.04 | 2.77±0.14 | 7.9±0.73 |
| 3 | 23.3 ±1.4* | 23.5±0.57* | 16.11±0.34* | 10.4±0.77* |
p<0.05 in One-way ANOVA with post hoc tukey’s test.
The results showed that, the oxidative damage caused in Group 2 was reversed significantly in Group 3 which was comparable to Group 1. One-way ANOVA test was performed for the oxidative stress markers between the groups showed that there was a statistically significant difference between groups (p<0.05).
Results for Neurotransmitter Levels
The mean and standard error for 5HT, NE and DA levels were calculated and tabulated in [Table/Fig-4].
The levels of neurotransmitters in brain.
| Group | Serotonin (ng/ml) | Nor-Epinephrine (ng/ml) | Dopamine (ng/ml) |
|---|
| 1 | 852.1±13.8 | 464.03±19.09 | 675.5±29.5 |
| 2 | 705.5±43.1 | 367.09±25.21 | 564.1±30.03 |
| 3 | 866.8±51.1* | 442.561±15.65* | 700.5±14.8* |
p<0.05 in One-way ANOVA with post hoc turkey’s test.
The results showed that administration of fluoxetine during cold restraint stress significantly improved the levels of all three neurotransmitters in brain. The level of serotonin was high when compared to the other two neurotransmitters. One-way ANOVA test was performed for neurotransmitter levels between the groups and showed that there was a statistically significant difference between groups (p<0.05).
Corticosterone Hormone Estimation
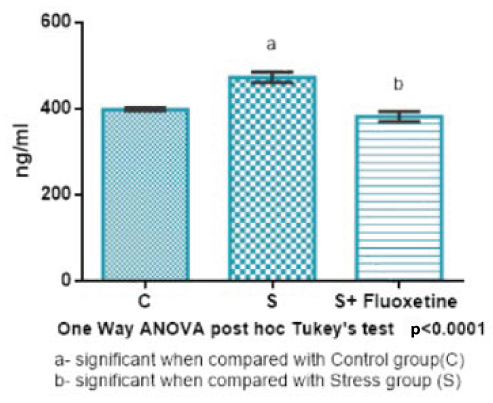
Serum corticosterone hormone levels [Table/Fig-5] were estimated in ng/ml and the values are expressed in terms of mean and standard deviation. In Group 1 (control) is 397.6±3.54, in Group 2 (cold restraint stress) was 471.8±12.2, in Group 3 (cold restraint stress with fluoxetine 10 mg/kg) was 381.3±11.5. One-way ANOVA test was performed for corticosterone hormone levels between the groups and showed that there was a highly statistically significant difference between groups (p<0.001).
Bar diagram showing the mean and SEM of level of serum corticosterone hormone in rats (n=6).
Cell Counting in H&E Staining
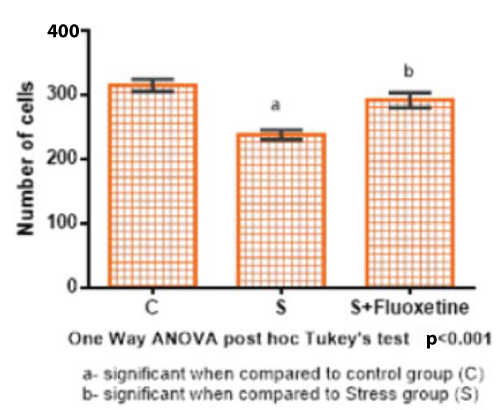
The total number of pyramidal cells in the CA3 region was expressed in mean and standard error [Table/Fig-6]. In Group 1 (Control) was 315.5±9.6, in Group 2 (cold restraint stress) was 238.3±7.5, in Group 3 (cold restraint stress with fluoxetine 10 mg/kg) was 292.1±11.3. One-way ANOVA test was performed for cell counts between the groups and showed that there was a highly statistically significant difference between groups (p<0.001) [Table/Fig-7,8 and 9].
Bar diagram showing the mean and SEM of total number of cells in CA3 region (n=6).
Group 1- Black arrow shows normal neuron with Pin-point nucleus, (100X) stained with H&E in CA3 region.
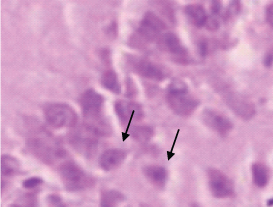
Group 2 – Black arrow shows normal neuron with Pin-point nucleus, Green arrow shows neuron with distorted morphology (100X) stained with H&E in CA3 region. Note: the increase in the number of distorted neurons.
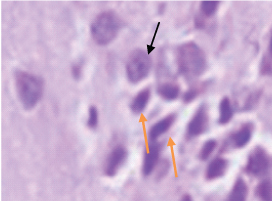
Group 3- Black arrow shows normal neuron with Pin-point nucleus, Green arrow shows neuron with distorted morphology (100X) stained with H&E in CA3 region. Note: the decrease in the number of distorted neurons
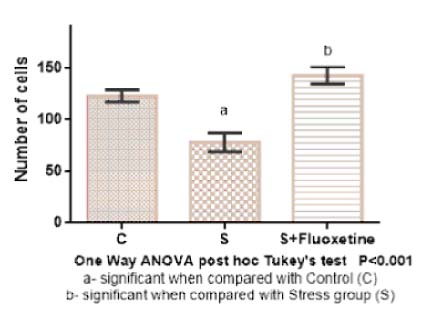
Bar diagram showing the mean and SEM of total number BrdU positive cells in SGZ (SubGranular Zone) region (n=6).
BrdU Positive Cell Counting
The total number of BrdU positive cells in the SGZ were counted and expressed in terms of means and standard error [Table/Fig-10]. In group 1 (control) was 120.2±3.5, in Group 2 (cold restraint stress) was 78.4±9.3, in Group 3 (cold restraint stress with fluoxetine 10 mg/kg) was 143.1±8.1. One-way ANOVA test was performed for BrdU positive cell counts between the groups and showed that there was a highly statistically significant difference between groups (p<0.001) [Table/Fig-11,12 and 13].
A 40x BrdU labelled image in SGZ of hippocampus in Group 1 (Control group), Black Arrow shows BrdU labelled cell.
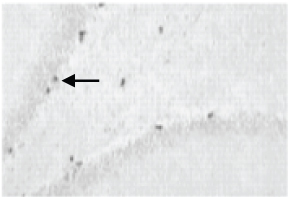
A 40x BrdU labelled image in SGZ of hippocampus in Group 2 (cold restraint stress), Black arrow shows a Neuron labelled with BrdU, Note the decrease in the number of BrdU labelled cell.
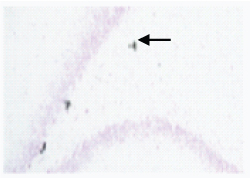
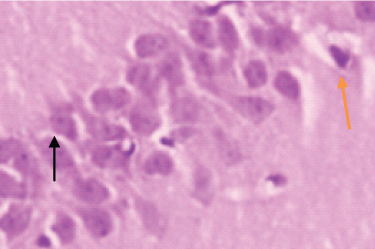
A 40x BrdU labelled image in SGZ of hippocampus in Group 3 (Cold restraint stress + Fluoxetine 10mg/kg) Black arrow shows a Neuron labelled with BrdU, Note the increase in the number of BrdU labelled cell, Orange arrow shows cluster of BrdU labelled cell.

Discussion
Stressful events can affect digestion, immune responses, endocrine function, brain function, behaviour, and cognition [13-15]. Animal and human studies have revealed that stress and its related pathology are related to both structural and functional changes in the brain, mainly in the hippocampal circuitry. In this study, the stress paradigm consists of cold stress, immobilisation stress and change in environment where the animals were moved from one experimental room to the other room.
Our studies showed that, all the antioxidant levels were statistically significant in Group 3 when compared to Group 2. Thus, fluoxetine improved the levels of antioxidants significantly post stress. Study by Novio S et al., showed that, there was an increase in the Reactive Oxygen Species (ROS) in the stressed group and treatment with fluoxetine reversed the effect through the improvement in the level of antioxidants like SOD, CAT and GSH [16]. The SOD and GSH has inhibitory effects on cellular apoptosis which are initiated by oxidative damage [17,18]. At a molecular level, studies by Thome J et al., showed that, antidepressant drugs like fluoxetine upregulate cAMP Response Element Binding (CREB) Protein by genomic action leading to enhanced gene expression of these antioxidants [19].
Our studies showed that fluoxetine reverses the behavioural deficits which were noted after the animals were treated with a combination of stress and fluoxetine 10 mg/kg given for a period of five weeks with one week of pre-treatment. The time taken for the rat to reach the platform was significantly lower when compared to Group 2. Similar reports were also reported in studies by Yau JL et al., where antidepressant treatment reversed the behavioural deficits in stressed animals [20].
Fluoxetine is a selective serotonin reuptake inhibitor drug which is used to treat major depressive disorders like impaired recall and memory deficits [21]. The probable mechanism for this reversal of the behavioural deficit may be due to the regulation of the corticosterone levels and upregulation of brain derived neurotrophic factor and CREB [22]. However, a similar study by Gumuslu E [23] showed that fluoxetine had only a partial improving effect in water maze test. There was impaired spatial learning in animals treated with selective serotonin reuptake inhibitor [24]. This may due to test dependency or may be due to high dosage which may be evident from human trials [23,25].
In the present study, neurotransmitters such as 5HT, DA, NE was decreased significantly in Group 2 when compared to Group 1. Cold restraint stress decreased the monoaminergic neurotransmitters in the hippocampus of the rats. Chronic stress leads to alteration in the monoaminergic neurotransmitter in the brain. In ventral hippocampus, there was decrease in 5HT which also affects the neurogenesis in that part of the brain [26]. The data available on the level of dopamine after stress is less. There was increase in the dihydroxyphenylacetic acid levels (metabolite of DA) in the prefrontal cortex, corpus striatum and brain stem after foot shocks for 10 days [27,28]. However, in our study, there was decrease in the DA level after cold restraint stress.
In our study there was increase in the level of DA, 5HT, NE in the brain of Group 3. Thus it can be clearly seen that, the level of monoaminergic neurotransmitter has been restored in Group 3 when compared to Group 2. This shows that the restoration of the levels of the above said neurotransmitters may be involved in the antidepressant effect of fluoxetine. Fluoxetine was found to increase the 5HT levels in the striatum with no changes in the levels of DA [29]. Another study has shown that, apart from increasing the extracellular levels of 5HT in the prefrontal cortex, fluoxetine was also found to increase the levels of DA and NE [30] which is similar to our study where there was an increase in serotonin, NE and dopamine.
Our study shows that the level of serum corticosterone hormone was elevated significantly in Group 2 when compared to the control group (p<0.001). Exposure of animals to chronic cold restraint stress has been suggested to upregulate the stress hormones by hyper activation of Hypothalamic-Pituitary-Adrenal (HPA) axis which can alter antioxidant and monoaminergic systems [31]. Studies by Liang S et al., showed that chronic restraint stress model increased the levels of cortisol in blood [32]. The hippocampus serves as both a target and a regulator of the brain’s response to stress.
In the present study, there was a decrease in the serum corticosterone level in Group 3 treated with 10 mg /kg of fluoxetine after cold restraint stress. When compared to the stressed group, there was significant decrease in the levels of corticosterone. This shows that treatment with fluoxetine can have an antistressor effect partly due to the reduction on the circulating levels of corticosterone. Studies have shown that fluoxetine decrease the level of corticosterone in groups that were injected with a dose of 40 mg/kg. Since elevated corticosterone is considered as a depression model, administration of 20 mg/kg of fluoxetine in that study reduced the hormone level significantly (p<0.001) [33].
Studies in humans have also supported similar results where, patients who were treated successfully with fluoxetine for depression had lower levels of cortisol when compared to those patients where the treatment with fluoxetine was not effective. Thus, it is clear that the beneficial therapeutic wellness after treatment is also due to decrease in the level of stress hormone [34]. At a molecular level, studies have shown that fluoxetine increases the mineralocorticoid receptor [35,36] and also glucocorticoid receptor [37] which is associated with enhanced negative feedback regulation, and thus with decreased resting and stimulated HPA axis activity [38] leading to decrease in the level of corticosterone hormone.
From the results, it was observed that there is an increase in the number of BrdU positive cells in Group 3 when compared to group 2. The number of BrdU positive cells compared to the control group was less but there was significant recovery of cells in Group 3 when treated with fluoxetine after cold restraint stress. It was found in the study that a dose of 10 mg/kg produced increase in brain derived neurotrophic factor and hippocampal neurogenesis but not in lower doses. Long term treatment with fluoxetine more than six weeks has been known to produce a reversal effect on neurogenesis in the Subventricular Zone (SVZ) but no such reversal phenomenon was noted in the dentate gyrus region [39]. However, the decrease of neurons in the SVZ may have a role in the side effects of fluoxetine.
In our study, the rats were treated with fluoxetine for a period of five weeks. This five week period produced remarkable recovery of BrdU positive cells in the SGZ region hippocampus. Fluoxetine also increased neurogenesis in hippocampal dentate gyrus post global transient ischemia [40]. It also improved neurogenesis post traumatic brain injury at a dosage of 10 mg/kg after a period of four weeks [41].
In the present study, there was also an increase in number of pyramidal cells in the slides stained in H&E in Group 3 when compared to the negative control group (Group 2). This shows the neuroprotective action of the fluoxetine where it safeguards the CA3 cells. The morphology of the pyramidal cells in Group 3 was comparable with those in Control group (Group 1). Very recent study showed that accumulation of excitotoxic Ca2+ and Zn2+ leads to CA3 neuron injury and in case of sublethal injury CA3 neurons recovers better [42].
Studies have also shown selective vulnerability of neurons in CA3 and CA1 region when compared to other areas of hippocampus caused mainly by increased intracellular accumulation of calcium inside those vulnerable neurons [43]. CA3 neurons are more damaged when compared to CA1 neurons despite its deep position inside hippocampus [44]. This clearly shows that the CA3 region along with CA1 region is more vulnerable to hippocampal injury. Our study had a similar finding in case of chronic restraint stress model treated with 10 mg/kg of fluoxetine.
cAMP, brain derived neurotrophic factor have been found to be upregulated in antidepressant treatment [45-48]. Another probable mechanism has been reported with increase in the activity of 5HT1A receptor function [49,50]. The increase in the number of proliferating neurons in the hippocampus may be also one of the therapeutic mechanisms for the action of fluoxetine. Thus, the improvement in the behaviour effects noted in our studies can be attributed to the increase in neurogenesis as one of the key factors.
Limitation
The present study was done with a single dose of 10 mg/kg. In future, a higher dosage could be used as an additional group to compare if there is a significant dose related therapeutic benefits in the animal model and compare it efficacy with an herbal drug.
Conclusion
Treatment with fluoxetine in cold restraint stress model significantly reversed the behavioural deficits in our animal model studies. This probably would have been due to its role in decreasing the corticosterone and improving the levels of antioxidants and neurotransmitters in the brain which may have caused an increase in the number of BrdU positive cells in the SGZ region of the hippocampus and also protecting the already existing pyramidal cells in CA3 region. Studies conducted previously have shown almost 75% of the total number of BrdU positive cells differentiates into mature neurons and the remainder differentiates into astrocytes and non specific cells which show that neurogenesis may contribute for the antistressor effects in our study. All these vital factors may be responsible for its therapeutic benefits.