Renal pelvis leiomyomas are infrequent benign tumours. These tumours are more frequent in women, usually asymptomatic and difficult to distinguish from malign kidney masses. A 27-year-old female presented with an asymptomatic renal mass discovered after abdominal ultrasound during routine check-up. Percutaneous renal biopsy was performed and reported urothelial carcinoma. After open nephroureterectomy, histopathological evaluation and immunohistochemistry were positive for Smooth Muscle Actin (SMA), Ki67 <5%, and negative for cytokeratin and HMB-45. Thus, confirming the diagnosis of renal leiomyoma. The diagnosis of these infrequent tumours is often difficult and it is usually made by immunohistochemistry after surgical treatment.
Case Report
A 27-year-old female presented with a history of two months of an asymptomatic renal mass. There was no relevant previous medical history and no family history of neoplasms. She consulted because two months ago an abdominal and renal ultrasonography were performed by her primary care physician during routine check-up and a renal mass was evidenced. With these findings she was referred to urological evaluation without primary treatment or further radiologic evaluation. The first urologist performed a percutaneous renal biopsy according to ultrasonography findings. The diagnosis of urothelial carcinoma was made after histological evaluation.
The patient looked for a second opinion and during evaluation in our clinic, the patient had no history of significant weight loss, anorexia, fever, hypertension, urinary tract infections, haematuria or gastrointestinal symptoms.
Physical examination did not evidence pain during right flank palpation, no palpable masses or peritoneal reaction was present. Laboratory findings from routine blood tests (haemoglobin, white cell count, platelets, creatinine, liver function test, coagulation) and urinalysis were normal.
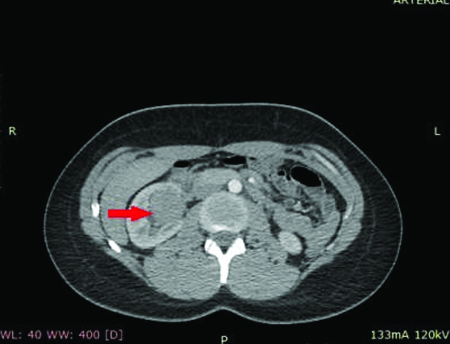
An abdominopelvic Contrast Enhanced Computed Tomography (CECT) was performed. Without contrast, the CT revealed a well-defined limit, solid mass (45-55 HU) located in topography of the renal pelvis. After contrast administration the CT demonstrated a >20 HU enhancement. No abdominal lymphadenopathy was reported [Table/Fig-1].
Radiological appearance of renal leiomyoma showing solid density and contrast enhancement after intravenous contrast administration.
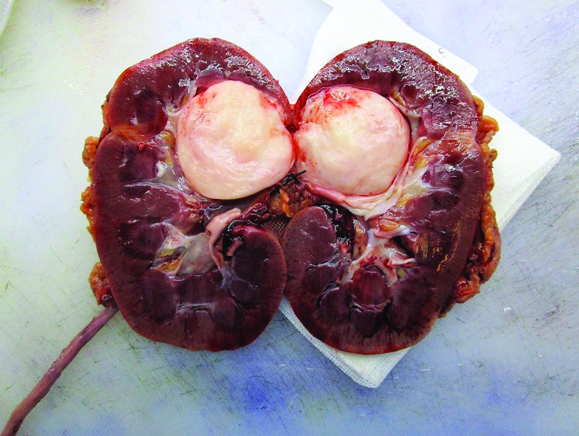
These findings were consistent with a renal mass suspicious for malignancy thus, open nephroureterectomy was defined as treatment [Table/Fig-2]. No complications were present during or after surgery. Macroscopic examination of the surgical specimen showed a 4 x 3 x 2 cm pink, roughed, renitent, round, well circumscribed, encapsulated tumour within the renal pelvis and did not appear to infiltrate the kidney [Table/Fig-3]. Microscopic evaluation revealed bundles of spindle cells with a fascicular growth pattern. There were no epithelial components, no cellular pleomorphism, haemorrhage or necrosis.
Nephroureterectomy specimen without relevant superficial changes.

Renal mass with solid aspect located within the renal pelvis.
After immunohistochemical testing, tumour cells expressed desmin, smooth muscle actin and h-caldesmon, while HMB-45 and cytokeratin AE1/AE3 were negative. Ki-67 of less than 5% correlated with a low mitotic index. Morphological features and immunohistochemistry profile were consistent with renal leiomyoma.
Discussion
Renal leiomyomas are extremely rare benign tumours. In a review performed by the Brady Urological Institute in 2005 of 1030 consecutive nephrectomies over a 10-year period, renal leiomyomas accounted for 1.5% of the benign lesions and 0.3% of all nephrectomies specimens [1].
Leiomyomas occur more often in women, Caucasian population, between the second and fifth decades of life and affection to both kidneys have been equally reported. There are usually asymptomatic but approximately 50% of patients present with palpable mass, abdominal or flank pain, and only 20% present with gross haematuria [2].
Three groups of patients have been described according to their clinical features: (1) small, multifocal, asymptomatic, incidentally detected tumours during routine exams, autopsy or after radical nephrectomy; (2) big, single and symptomatic tumours (discovered secondary to abdominal or flank pain, palpable mass or haematuria); and (3) large tumours detected radiographically, but without clinical signs and symptoms [3].
In our case, the patient’s presentation was the most common (asymptomatic and tumour discovered during routine exams) and compatible with group 1 features.
Renal leiomyomas arise from the mesenchymal tissue (muscle cells) of different structures of the kidney, most frequently in the renal capsule and less commonly in the renal pelvis and renal vessels [4].
Renal pelvis location of renal leiomyomas is unusual. According to Steiner MS et al., within symptomatic leiomyomas, tumour localization is subcapsular in 53%, capsular in 37% and renal pelvis in less than 10% [5].
Radiologic evaluation of renal masses often includes CECT and Magnetic Resonance Imaging (MRI). Radiologic features of renal leiomyomas in CECT are non-specific, but these tumours usually exhibit contrast enhancement, well defined borders, and they cannot be precisely differentiated from other renal tumours. In MRI these lesions tend to be homogeneous, with nodular areas, isointense and hypointense to renal cortex on T1 and T2-weighted images with peripheral gadolinium enhancement [6].
In this case, after contrast administration the CT demonstrated a >20HU enhancement and a filling defect within the renal pelvis, therefore nephroureterectomy was performed.
Leiomyoma is clinically and radiologically indistinguishable from other renal neoplasms. For these reasons, definitive diagnosis is usually made after pathological examination and it is usually confirmed after immunochemistry evaluation. The differential diagnosis includes angiomyolipoma, oncocytoma, renal cell carcinoma, adult mesoblastic nephroma and leiomyosarcoma [7].
In immunochemistry profile angiomyolipomas usually express HMB-45, a melanocytic marker; epithelial and stromal tumours (mesoblastic nephroma and renal cell carcinoma) show to be immunoreactive with antibodies to cytokeratin and epithelial elements and renal leiomyomas are often positive to SMA [8].
In our case, the immunochemistry profile was compatible with these markers and h-caldesmon and desmin were also positive as described in other cases [9].
By the fact that an accurate preoperative diagnosis is difficult to achieve, renal leiomyomas are usually treated invasively. Indications for surgery (open, laparoscopic, partial or total nephrectomy) include: symptomatic lesions, especially weight loss, haematuria, evidence of tumour growth and suspicion of sarcomatous degeneration [10]. In the present case, a previous malignant diagnosis was given and CT showed suspicious radiologic behaviour, thus justifying the invasive treatment.
Conclusion
Renal leiomyomas are very infrequent tumours. An accurate preoperative diagnosis according to clinical or radiological features is difficult to obtain and diagnosis is often made by immunohistochemistry after surgical treatment.
[1]. Romero F, Kohanim S, Guilherme L, Permpongkosol S, Fine SW, Kavoussi LR, Leiomyomas of the kidney: emphasis on conservative diagnosis and treatmentUrology 2005 66(6):1319.e1-19.e3. [Google Scholar]
[2]. Andreoiu M, Drachenberg D, MacMahon R, Giant renal leiomyoma: a case report and brief review of the literatureCan Urol Assoc J 2009 3(5):E58-E60. [Google Scholar]
[3]. Brunocilla E, Pultrone CV, Schiavina R, Vagnoni V, Caprara G, Martorana G, Renal leiomyoma: Case report and literature reviewCan Urol Assoc J 2012 6(2):E87-E90. [Google Scholar]
[4]. Fu L, Humphrey PA, Adeniran AJ, Renal leiomyomaJ Urol 2015 193(3):997-98. [Google Scholar]
[5]. Steiner MS, Quinlan D, Goldman SM, Millmond S, Hallowell MJ, Stutzman RE, Leiomyoma of the kidney: presentation of 4 new cases and the role of computerized tomographyJ Urol 1990 143:994-98. [Google Scholar]
[6]. Goren MR, Erbay G, Ozer C, Goren V, Bal N, Bilateral renal leiomyoma with 5 year follow-up: Case reportCan Urol Assoc J 2015 9(9-10):E734-E736. [Google Scholar]
[7]. Hogan A, Smyth GK, D’Arcy C, O’Brien A, Quinlan DM, Renal capsular leiomyomaUrology 2008 71:1226.e1-1226.e3. [Google Scholar]
[8]. Khetrapal S, Bhargava A, Jetley S, Rana S, Jairajpuri Z, Renal leiomyoma: an uncommon differential diagnosis of renal masses with a clinical relevanceJCDR 2014 8(10):FD08-FD09. [Google Scholar]
[9]. Kuroda N, Inoue Y, Taguchi T, Tominaga A, Hes O, Michal M, Renal leiomyoma: an immunohistochemical, ultrastructural and comparative genomic hybridization studyHistol Histopathol 2007 22(8):883-88. [Google Scholar]
[10]. Romero F, Kohanim S, Guilherme L, Permpongkosol S, Fine SW, Kavoussi LR, Leiomyomas of the kidney: emphasis on conservative diagnosis and treatmentUrology 2005 66(6):1319.e1-1319.e3. [Google Scholar]