Denture stomatitis affects almost 15 to 65% of healthy mouths who are denture wearers [1]. The aetiology of denture stomatitis is generally multifactorial which includes tissue trauma from ill fitting dentures, lack of denture cleanliness, dietary factors, continuous denture use without removal, chronic illness, and a compromised and poor immune system. Predisposing factors are inadequate denture hygiene, denture wearing habits, xerostomia, medications and nutritional factors [2,3]. Although Candida albicans has been established as a primary aetiologic agent, denture stomatitis is not a result of Candida albicans solely but rather it is an outcome of multispecies biofilms that may include Streptococcus mutans [4]. It is seen that coadhesion between Candida albicans and many Streptococcal species helps in oral colonisation by yeast cells [5].
The initial steps taken to prevent and treat denture stomatitis are by improving denture adaptation and preparing the conditions for recovering of denture bearing tissues. But tissue conditioners that are used to treat the abused denture bearing areas are more prone to colonisation by micro-organisms as compared to denture base resins [6]. Colonisation of Candida results in the formation of Candidal biofilm on the surfaces of tissue conditioner in an aqueous environment. This is followed by microbial surface attachment, cell proliferation, matrix production and detachment [7].
In recent studies the use of antimicrobial agents in tissue conditionershas attracted interest to control the micro-organisms [8,9]. The advantage of incorporation of these agents into the tissue conditioner can reduce the limited compliance of the patient and cost of treatment.
The purpose of this study was to compare the efficacy of neem extract and three antimicrobial agents incorporated in a tissue conditioner against Candida albicans and Streptococcus mutans.
Materials and Methods
This in vitro study was carried out in the Department of Prosthodontics and the Department of Microbiology at Saraswati Dental College and Hospital, Lucknow (Uttar Pradesh).
Candida albicans and Streptococcus mutans inoculation preparation: Standard strains of Candida albicans (ATCC 10231, HIMEDIA) and Streptococcus mutans (ATCC 25175, HIMEDIA) were inoculated into Sabouraud Dextrose broth and Mitis-Salivarius-Bacitracin respectively and incubated at 37°C for 24 hours. After that Candida albicans and Streptococcus mutans were standardised by dilution with sterile broth. Then 0.5 ml of diluted Candida albicans and Streptococcus mutans were dropped on each sterile Sabourads agar and Mitis-Salivarius-Bacitracin plates respectively and lawn cultures were made.
Incorporation of Antifungals into Tissue Conditioner: After the inoculums dried, a well of 6 mm diameter was punched in 5 mm deep agar plate with a sterile punch cork borer. Four holes were punched in each plate for a given concentration. Ketoconazole (HIMEDIA), nystatin (HIMEDIA), chlorhexidine diacetate powder (HIMEDIA) (5% w/w and 10% w/w) and neem leaf extract (Forest Research Institute, Dehradun) (7.5% and 15% w/w) were hand mixed with tissue conditioner powder (Viscogel, Dentsply, USA) for 30 seconds according to manufacturer’s instructions. A wooden stick was used to carry the samples into the punch holes (diameter=6 mm) in the inoculated petriplates. Plates were incubated at 37°C for seven days. MID for each test punch hole was measured in millimetres across the punch hole after 24 hours and seven days using a vernier calliper. Triplicates were done of each concentration to check the repeatability of the antimicrobial effect.
Twelve samples were taken for each concentration, so a total of 108 samples were taken for Candida albicans and divided into nine groups as follows:
Group1–Neem leaf extract with Viscogel tissue conditioner at 7.5% w/w
Group2–Neem leaf extract with Viscogel tissue conditioner at 15% w/w
Group3–Ketoconazole with Viscogel tissue conditioner at 5% w/w
Group4–Ketoconazole with Viscogel tissue conditioner at 10% w/w
Group5 Nystatin with Viscogel tissue conditioner at 5% w/w
Group6–Nystatin with Viscogel tissue conditioner at 10% w/w
Group7–Chlorhexidine diacetate with Viscogel tissue conditioner at 5% w/w
Group8–Chlorhexidine diacetate with Viscogel tissue conditioner at 10% w/w
Group9–Control group which contained plain tissue conditioner without any antimicrobial agent.
Similarly, a total of 108 samples were taken for Streptococcusmutans and divided into nine groups.
Descriptive statistics were presented in the form of mean, standard deviation, median and quartiles. At each time interval, the inhibition zones between different study groups were compared using Kruskal Wallis test followed by Mann-Whitney U test as post hoc test for pair wise comparison. In each group, the inhibition zones at two time intervals were compared using wilcoxon sign rank test. P<0.05 was considered to be statistically significant.
Results
The efficacy of four antimicrobial agents against Candida albicans and Streptococcus mutans were determined by measuring the MID after 24 hours and seven days at two different concentrations and the results are summarised in [Table/Fig-1,2,3,4,5 and 6].
Statistical analysis of MID of different concentrations (% w/w) of neem extract, ketoconazole, nystatin and chlorhexidine diacetate in Viscogel tissue conditioner and without any microbial agents against Candida albicans using Kruskal Wallis test.
| Group -Candida albicans | | 24 Hours | 7 Days | Wilcoxon sign rank test |
|---|
| N | Mean (SD) | Median (Q1-Q3) | Mean (SD) | Median (Q1-Q3) | z | p-value |
|---|
| 1- Neem extract (7.5%) | 12 | 9.50 (0.67) | 10 (9-10) | 8.33 (0.77) | 8.50 (8-9) | -2.72 | 0.006* |
| 2- Neem extract (15%) | 12 | 20.67 (0.49) | 21 (20-21) | 17.75 (0.45) | 18 (17.25-18) | -3.13 | 0.002* |
| 3 – Ketoconazole (5%) | 12 | 31.42 (0.79) | 32 (31-32) | 30.42 (0.51 | 30 (30-31) | -2.76 | 0.006* |
| 4– Ketoconazole (10%) | 12 | 31.75 (0.45) | 32 (31.25-32) | 31.50 (0.52) | 31.50 (31-32) | -1.34 | 0.18(NS) |
| 5 – Nystatin (5%) | 12 | 31.67 (0.49) | 32 (31-32) | 31.08 (0.79) | 31 (30.25-32) | -1.71 | 0.08(NS) |
| 6– Nystatin (10%) | 12 | 31.75 (0.45) | 32(31.25-32) | 30.92 (0.66) | 31(30.25-31) | -2.48 | 0.01* |
| 7- Chlorhexidine diacetate (5%) | 12 | 17.83 (0.38) | 18(18-18) | 17.17 (0.83) | 17(16.25-18) | -1.99 | 0.04* |
| 8- Chlorhexidine diacetate(10%) | 12 | 18.33 (0.49) | 18(18-19) | 17.50 (0.52) | 17.50 (17-18) | -2.42 | 0.02* |
| 9 - Control | 12 | 6(0) | 6(6-6) | - | - | - | - |
| Kruskal Wallis test | Chi-square value | 100.092 | 98.229 | |
| p-value | <0.001* | <0.001* |
p<0.05 statistically significant p>0.05 non significant, NS
-Pairwise comparison between groups against Candida albicans in each time interval using Mann-Whitney U test.
| Candida albicans | 24 Hours | 7 Days |
|---|
| U statistic | z | p-value | U statistic | z | p-value |
|---|
| 1 vs 2 | 0 | -4.31 | <0.001* | 0 | -4.32 | <0.001* |
| 1 vs 3 | 0 | -4.27 | <0.001* | 0 | -4.27 | <0.001* |
| 1 vs 4 | 0 | -4.34 | <0.001* | 0 | -4.26 | <0.001* |
| 1 vs 5 | 0 | -4.31 | 0.001* | 0 | -4.23 | <0.001* |
| 1 vs 6 | 0 | -4.34 | <0.001* | 0 | -4.25 | <0.001* |
| 1 vs 7 | 0 | -4.38 | <0.001* | 0 | -4.23 | <0.001* |
| 1 vs 8 | 0 | -4.31 | <0.001* | 0 | -4.26 | <0.001* |
| 1 vs 9 | 0 | -4.52 | <0.001* | 0 | -4.49 | <0.001* |
| 2 vs 3 | 0 | -4.30 | <0.001* | 0 | -4.35 | <0.001* |
| 2 vs 4 | 0 | -4.36 | <0.001* | 0 | -4.34 | <0.001* |
| 2 vs 5 | 0 | -4.33 | <0.001* | 0 | -4.30 | <0.001* |
| 2 vs 6 | 0 | -4.36 | <0.001* | 0 | -4.33 | <0.001* |
| 2 vs 7 | 0 | -4.41 | <0.001* | 43.50 | -1.86 | 0.06(NS) |
| 2 vs 8 | 0 | -4.33 | <0.001* | 54.00 | -1.23 | 0.21(NS) |
| 2 vs 9 | 0 | -4.55 | <0.001* | 0 | -4.59 | <0.001* |
| 3 vs 4 | 57.00 | -1.04 | 0.30(NS) | 15.00 | -3.53 | <0.001* |
| 3 vs 5 | 62.00 | -0.67 | 0.50(NS) | 38.00 | -2.12 | 0.03* |
| 3 vs 6 | 57.00 | -1.04 | 0.29(NS) | 43.00 | -1.86 | 0.06(NS) |
| 3 vs 7 | 0 | -4.37 | <0.001* | 0 | -4.26 | <0.001* |
| 3 vs 8 | 0 | -4.30 | <0.001* | 0 | -4.29 | <0.001* |
| 3 vs 9 | 0 | -4.51 | <0.001* | 0 | -4.53 | <0.001* |
| 4 vs 5 | 66.00 | -0.44 | 0.66(NS) | 51.00 | -1.33 | 0.18(NS) |
| 4 vs 6 | 72.00 | 0 | 1.00(NS) | 39.00 | -2.12 | 0.03* |
| 4 vs 7 | 0 | -4.44 | <0.001* | 0 | -4.25 | <0.001* |
| 4 vs 8 | 0 | -4.36 | <0.001* | 0 | -4.29 | <0.001* |
| 4 vs 9 | 0 | -4.59 | <0.001* | 0 | -4.52 | <0.001* |
| 5 vs 6 | 66.00 | -0.44 | 0.66(NS) | 63.00 | -.56 | 0.57(NS) |
| 5 vs 7 | 0 | -4.41 | <0.001* | 0 | -4.22 | <0.001* |
| 5 vs 8 | 0 | -4.33 | <0.001* | 0 | -4.25 | <0.001* |
| 5 vs 9 | 0 | -4.55 | <0.001* | 0 | -4.48 | <0.001* |
| 6 vs 7 | 0 | -4.44 | <0.001* | 0 | -4.24 | <0.001* |
| 6 vs 8 | 0 | -4.36 | <0.001* | 0 | -4.28 | <0.001* |
| 6 vs 9 | 0 | -4.59 | <0.001* | 0 | -4.51 | <0.001* |
| 7 vs 8 | 40.00 | -2.43 | 0.02* | 57.00 | -0.95 | 0.34(NS) |
| 7 vs 9 | 0 | -4.64 | <0.001* | 0 | -4.48 | <0.001* |
| 8 vs 9 | 0 | -4.55 | <0.001* | 0 | -4.52 | <0.001* |
p<0.05 statistically significant, p>0.05 non significant, NS
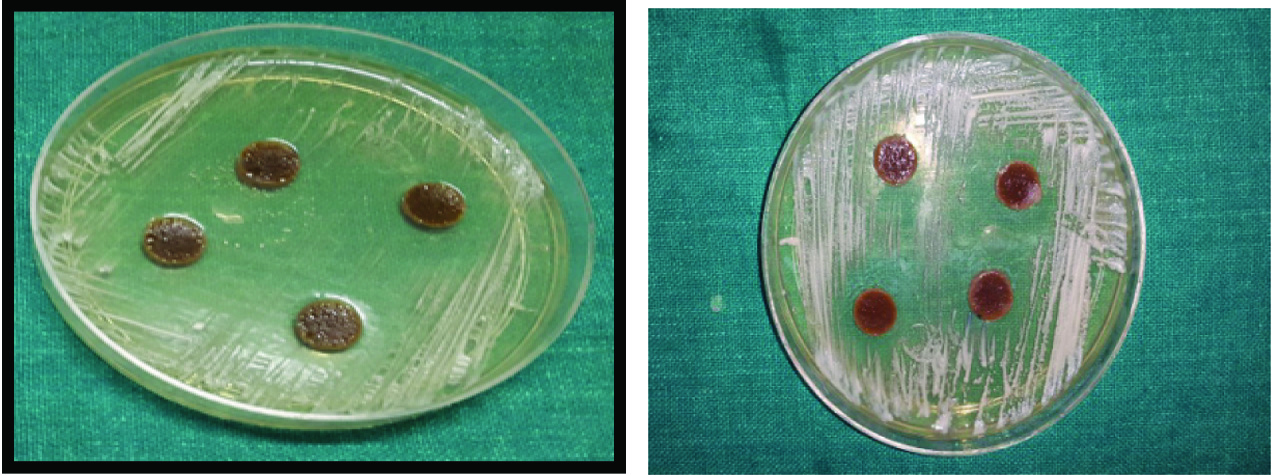
(a) Inhibition of C. albicans with 15% w/w neem extract with Viscogel after 24 hours; (b) Inhibition of C. albicans with 15% w/w neem extract with Viscogel after seven days. (Images left to right)
Statistical analysis of MID of different concentrations (% w/w) of neem extract, ketoconazole, nystatin and chlorhexidine diacetate against Streptococcus mutans in Viscogel tissue conditioner and without any microbial agents using Kruskal Wallis test.
| Group -Streptococcus Mutans | | 24 hours | 7 Days | Wilcoxon sign rank test |
|---|
| N | Mean (SD) | Median (Q1-Q3) | Mean (SD) | Median (Q1-Q3) | z | p-value |
|---|
| 1- Neem extract (7.5%) | 12 | 21.83 (0.39) | 22 (22-22) | 21.25(0.43) | 21 (21-21.75) | -2.646b | .008 |
| 2- Neem extract (15%) | 12 | 23.67 (0.49) | 24 (23-24) | 23.33 (0.49) | 23 (23-24) | -2.00 | 0.046* |
| 3 – Ketoconazole (5%) | 12 | 6.00 (0) | 6 (6-6) | 6.00 (0) | 6 (6-6) | 0 | 1.00(NS) |
| 4– Ketoconazole (10%) | 12 | 6.00 (0) | 6 (6-6) | 6.00 (0) | 6 (6-6) | 0 | 1.00(NS) |
| 5 – Nystatin (5%) | 12 | 6.00 (0) | 6 (6-6) | 6.00 (0) | 6 (6-6) | 0 | 1.00(NS) |
| 6– Nystatin (10%) | 12 | 6.00 (0) | 6(6-6) | 6.00 (0) | 6(6-6) | 0 | 1.00(NS) |
| 7- Chlorhexidine diacetate (5%) | 12 | 24.17 (0.38) | 24(24 -24) | 24.08 (0.28) | 24(24 -24) | -1.000b | .317 |
| 8- Chlorhexidine diacetate(10%) | 12 | 25.67 (0.49) | 26(25-26) | 25.33 (0.49) | 25(25-26) | -2.000b | .046 |
| 9 - Control | 12 | 6(0) | 6(6-6) | - | - | - | - |
| Kruskal Wallis test | Chi-square value | 106.07 | 106.16 | |
| p-value | <0.001* | <0.001* |
p<0.05 statistically significant p>0.05 non significant, NS
Pairwise comparison between groups against Streptococcus mutans in each time interval using Mann-Whitney U test.
| S.Mutans | 24 hours | 7 Days |
|---|
| U statistic | z | p-value | U statistic | z | p-value |
|---|
| 1 vs 2 | 0 | -4.41 | <0.001* | 0 | -4.37 | <0.001* |
| 1 vs 3 | 0 | -4.63 | <0.001* | 0 | -4.58 | <0.001* |
| 1 vs 4 | 0 | -4.63 | <0.001* | 0 | -4.58 | <0.001* |
| 1 vs 5 | 0 | -4.63 | 0.001* | 0 | -4.58 | <0.001* |
| 1 vs 6 | 0 | -4.63 | <0.001* | 0 | -4.58 | <0.001* |
| 1 vs 7 | 0 | -4.49 | <0.001* | 0 | -4.41 | <0.001* |
| 1 vs 8 | 0 | -4.41 | <0.001* | 0 | -4.36 | <0.001* |
| 1 vs 9 | 0 | -4.63 | <0.001* | 0 | -4.58 | <0.001* |
| 2 vs 3 | 0 | -4.55 | <0.001* | 0 | -4.55 | <0.001* |
| 2 vs 4 | 0 | -4.55 | <0.001* | 0 | -4.55 | <0.001* |
| 2 vs 5 | 0 | -4.55 | <0.001* | 0 | -4.55 | <0.001* |
| 2 vs 6 | 0 | -4.55 | <0.001* | 0 | -4.55 | <0.001* |
| 2 vs 7 | 40.000 | -2.439 | .015 | 22.000 | -3.402 | .001 |
| 2 vs 8 | 0 | -4.34 | <0.001* | 0 | -4.34 | <0.001* |
| 2 vs 9 | 0 | -4.55 | <0.001* | 0 | -4.55 | <0.001* |
| 3 vs 4 | 72.00 | 0 | 1.00(NS) | 72.00 | 0 | 1.00(NS) |
| 3 vs 5 | 72.00 | 0 | 1.00(NS) | 72.00 | 0 | 1.00(NS) |
| 3 vs 6 | 72.00 | 0 | 1.00(NS) | 72.00 | 0 | 1.00(NS) |
| 3 vs 7 | 0 | -4.63 | <0.001* | 0 | -4.71 | <0.001* |
| 3 vs 8 | 0 | -4.55 | <0.001* | 0 | -4.55 | <0.001* |
| 3 vs 9 | 72.00 | 0 | 1.00(NS) | 72.00 | 0 | 1.00(NS) |
| 4 vs 5 | 72.00 | 0 | 1.00(NS) | 72.00 | 0 | 1.00(NS) |
| 4 vs 6 | 72.00 | 0 | 1.00(NS) | 72.00 | .0 | 1.00(NS) |
| 4 vs 7 | 0 | -4.64 | <0.001* | 0 | -4.71 | <0.001* |
| 4 vs 8 | 0 | -4.55 | <0.001* | 0 | -4.55 | <0.001* |
| 4 vs 9 | 72.00 | 0 | 1.00(NS) | 72.00 | 0 | 1.00(NS) |
| 5 vs 6 | 72.00 | 0 | 1.00(NS) | 72.00 | .0 | 1.00(NS) |
| 5 vs 7 | 0 | -4.64 | <0.001* | 0 | -4.70 | <0.001* |
| 5 vs 8 | 0 | -4.55 | <0.001* | 0 | -4.55 | <0.001* |
| 5 vs 9 | 72.00 | 0 | 1.00(NS) | 72.00 | 0 | 1.00(NS) |
| 6 vs 7 | 0 | -4.64 | <0.001* | 0 | -4.71 | <0.001* |
| 6 vs 8 | 0 | -4.55 | <0.001* | 0 | -4.55 | <0.001* |
| 6 vs 9 | 72.00 | 0 | 1.00(NS) | 72.00 | 0 | 1.00(NS) |
| 7 vs 8 | 4.00 | -4.193 | <0.001* | 4.00 | -4.264 | <0.001* |
| 7 vs 9 | 0 | -4.64 | <0.001* | 0 | -4.71 | <0.001* |
| 8 vs 9 | 0 | -4.55 | <0.001* | 0 | -4.55 | <0.001* |
p<0.05 statistically significant, p>0.05 non significant, NS
(a) Inhibition of S. mutans with 15% w/w neem extract with Viscogel after 24 hours; (b) Inhibition of S. mutans with 15% w/w neem extract with Viscogel after seven days. (Images left to right)

[Table/Fig-1] shows the inhibition diameter values of both ketoconazole and nystatin (5% and 10% w/w) confirming the highest fungicidal activity against Candida albicans after 24 hours as well as seven days. Pairwise comparisons are presented in [Table/Fig-2].
This was followed by Neem leaf extract (15% w/w) which showed maximum inhibition of 21 mm after 24 hours and 18 mm after seven days respectively [Table/Fig-3a,b].
[Table/Fig-4] shows MID of neem leaf extract, ketoconazole, nystatin and chlorhexidine diacetate in tissue conditioner and control group against Streptococcus mutans after 24 hours and after seven days at two different concentrations. Pairwise comparisons are presented in [Table/Fig-5].
[Table/Fig-6a,b] shows the inhibition zones of S. mutans after 24 hours and seven days.
Discussion
Denture stomatitis is an inflammatory process which affects the mucosa under dentures. Denture stomatitis has been classified based on their severity as Type I (Localised inflammation or pinpoint hyperaemia), Type II (More diffuse erythema involving part or all of the mucosa which is covered by the denture) and Type III (Inflammatory nodular/papillary hyperplasia usually on the central hard plate and the alveolar ridge), where Type III patients have the greatest severity of inflammation [13]. Although the aetiology is multifactorial, inflammation is primarily associated with Candidaalbicans, which is a normal oral commensal. Candidal invasion is further accelerated by trauma and the nature of the denture base, particularly if worn continuously and in the presence of poor oral hygiene. The biofilms also include Streptococcus mutans and Staphylococcus aureus [5].
Tissue conditioners have been successfully used to recondition the abused denture supporting tissues. Tissue conditioners that improve the health of abused denture bearing areas are more susceptible to colonisation by micro-organisms than denture base resins. In recent studies the use of antimicrobial agents in tissue conditioners has attracted interest to control the micro-organisms. Antifungal agents can be added to tissue conditioner to expedite recovery and may be a promising method of drug delivery to overcome obstacles of other therapies [14].
Synthetic or herbal antimicrobial agents are added to the tissue conditioner to reduce as well as to overcome the risk of getting this infection. Medicinal herbs are of interest especially in developing countries due to their effective antimicrobial activity, safety and affordability [15].
Microbial resistance to most of the antibiotics commonly used to treat oral infections (penicillins and cephalosporins, erythromycin, tetracycline and derivatives and metronidazole) has been documented. Due to reports of higher incidence of antibiotic misuse as well as overuse, biological and antimicrobiological properties of different plants have been studied [16].
In this study, agar punch well technique was used to evaluate and compare the efficacy of neem extract, ketoconazole, nystatin and chlorhexidine diacetate when incorporated as % weight/weight in tissue conditioner against Candida albicans and Streptococcus mutans. A 5% w/w and 10% w/w of ketoconazole, nystatin and chlorhexidine diacetate and 7.5% w/w and 15% w/w of neem leaf extract and plain tissue conditioner which acted as control group were tested at 24 hours and seven days of time interval to get a minimum most effective concentration by comparing the MID.
In case of Streptococcus mutans, the maximum inhibition was seen with 10% w/w chlorhexidine diacetate after 24 hours and seven days followed by 5% w/w chlorhexidine diacetate, 15% w/w and 7.5% w/w neem leaf extract. No inhibition was seen with both the concentrations of ketoconazole and nystatin as well as the control group.
In case of Candida albicans, both ketoconazole and nystatin (5% w/w and 10% w/w) when mixed with tissue conditioner showed maximum antifungal activity resulting in complete inhibition of growth of Candida albicans both after 24 hours as well as seven days. These results were consistent with the study done by Quinn DM who concluded that ketoconazole was as effective as nystatin in completely inhibiting the growth of Candida albicans and Thomas CJ and Nutt GM, who concluded that Nystatin was more effective in completely inhibiting the growth of Candida spp than Amphotericin B [17,18]. This was followed by 15% w/w of neem leaf extract which showed a maximum inhibition of 21 mm after 24 hours and minimum of 17 mm after seven days. Chlorhexidine diacetate (10% w/w) showed maximum inhibition of 19 mm after 24 hours and minimum of 17 mm after seven days. Other studies have also reported antifungal activity of neem against Candida albicans [19,20]. But there is no study where neem was incorporated into tissue conditioner as a mode of drug delivery against Candidaalbicans.
Present study demonstrated that neem leaf extract at 15% w/w exhibited good antifungal as well as antibacterial effect against Candida albicans and Streptococcus mutans after 24 hours and seven days. Bokhora A and co-workers concluded that neem leaf extract has a significant antimicrobial effect against Candida albicans and Rajsekharan C et al., concluded that Neem exhibited significant antibacterial effect against Streptococcus mutans [21,22]. Ketoconazole and nystatin had the best fungicidal activity but it showed no inhibition in case of Streptococcus mutans. Even though chlorhexidine is found to be a good antimicrobial agent, its adverse effects on long term usage are under scrutiny. Genotoxic and mutagenic properties and rarely severe contact dermatitis have been reported. Hence, it has been suggested to be used as a therapeutic agent and not for routine long term use [23]. Emerging drug resistance is also a major concern. Conventional drugs usually provide effective antimicrobial therapy but there is an increasing problem of microbial resistance and undesirable side effects [16].
Taking the above mentioned facts into consideration, it is tempting to speculate that in future neem could be used as a key ingredient to treat denture stomatitis. neem is not toxic and also does not have any mutagenic properties. Natural products have been found to have antibacterial and antifungal activities as well as anti-inflammatory and antioxidant effects, and also proven to be effective alternative substitutions of chemical substances with very less adverse effects [12]. The major benefits of using neem are its easy availability, cost effectiveness, good shelf life, low toxicity and no resistance of micro-organisms has been reported so far [24].
Limitation
The present study is limited to only one brand of tissue conditioner. Hence the results obtained here may not be applicable to other tissue conditioners incorporated with the same concentration of neem extract, ketoconazole, nystatin and chlorhexidine diacetate. There is scope for researches to conduct studies on other different brands of tissue conditioners incorporating the same concentrations of the antimicrobial agents. Further studies are required to determine the half life of the antimicrobial agents after it is mixed with tissue conditioners and the rate of release of these antimicrobial agents from it. Also, the effect of addition of antimicrobial agents especially neem on the desirable properties of tissue conditioners must be evaluated in future.
Conclusion
Both 5% w/w & 10% w/w ketoconazole and nystatin showed maximum antifungal effect against Candida albicans which is followed by 15% w/w neem leaf extract after both 24 hours as well as seven days. A 10% w/w chlorhexidine diacetate showed maximum antibacterial effect which is followed by 5% w/w chlorhexidine and 15% w/w neem leaf extract after 24 hours and seven days against Streptococcus mutans. Thus, 15% Neem leaf extract showed potential antimicrobial efficacy against both Candida albicans and Streptococcus mutans after 24 hours and seven days. Neem is easily available and cheap. Also, it has a good shelf life, low toxicity and no microbial resistance reported so far. Neem leaf extract may be used as the treatment of choice by incorporating into tissue conditioners in case of denture stomatitis.
*p<0.05 statistically significant p>0.05 non significant, NS
*p<0.05 statistically significant, p>0.05 non significant, NS
*p<0.05 statistically significant p>0.05 non significant, NS
*p<0.05 statistically significant, p>0.05 non significant, NS