OSMF is a premalignant condition of oral mucosa seen in individuals with habit of areca nut chewing. It is an insidious, chronic, irreversible progressive disease in which the symptoms vary from burning of oral mucosa to inability in opening mouth, difficulty in swallowing and speech. It is more prevalent in South East Asia and people of South Asian origin [1]. The possible etiological factors are areca nut and chillies [2], pan (betel leaf with tobacco powder and other ingredients) and alcohol. However, OSMF pathogenesis is likely to be multifactorial as only small proportion of nut users suffer from this condition [3]. Histopathologically OSMF is manifested by loss of rete ridges, flat epithelium connective tissue interface, epithelial atrophy, reduction in vascularity, chronic inflammatory infiltrate and fibrosis of connective tissue leading to subepithelial hyalinization. The level of vascularity of the OSMF affected mucosa and its effect on the epithelial thickness in OSMF is arguable [1].
Malignancy develops in about 14% of OSMF lesions; dysplasia is seen in 26% of OSMF lesions which is consistent with the high rate of malignant transformation. The epithelial atrophy occurs in 87% and keratinizing metaplasia in 67% of cases. Given these morphological changes, it is reasonable that the epithelium may play a role in inducing the connective tissue changes seen in OSMF and is certainly involved in the malignant changes that occur in a significant number of cases [3].
Along with repair process, inflammation induced vascular response is noted in OSMF. Same sections of OSMF show varied vasculature scenario ranging from normal, dilated to constricted blood vessels and a combination of both is also seen. The narrowing of the vessels is seen initially in superficial mucosa and in later stages, it spreads to deeper connective tissue. Presence of dilated vessels in advanced stages of OSMF is reported, altogether making the vascularity in OSMF a matter of conjuncture [4].
CD34 is a transmembrane glycoprotein present on endothelial cells, leukemic cells, and some progenitor cells, which is produced by endothelial cells and associated with angiogenesis. CD34 antigen is a sensitive marker for the vascular endothelium of both benign and neoplastic tissues [5].
Studies on microvasculature till date have not shown similar results. With this view in mind, present study was designed to investigate the mucosal vasculature status in OSMF using CD34 antibody, which is sensitive marker of vascular endothelium.
Materials and Methods
Samples
A retrospective study was designed by collecting samples from archives of Department of Oral and Maxillofacial Pathology from Modern Dental College and Research Center, Indore, Madhya Pradesh, India, and Sharad Pawar Dental College, Sawangi (M), Wardha, Maharashtra, India, between January 2015 and December 2016. The Scientific and Ethics Committee of The Institutes had approved this study. The staging of OSMF was performed based upon modification of Lai et al classification into Stage I, II and III [6]. In Stage I, interincisal opening was more than 30 mm, in Stage II between 30-21 mm and in Stage III less than 20 mm. Paraffin embedded specimens of 45 OSMF cases in total with biopsy site of buccal mucosa were selected from the archives. Fifteen tissue blocks of the normal buccal mucosa obtained from healthy individuals using punch biopsy were included. All the samples were subjected to immunohistochemical analysis for CD34. Demographic and clinical data for all the OSMF cases were retrieved from the archival records available in the department.
Immmunohistochemistry
For assessment of vascularity, we used 4 µm thick sections placed on 3-(triethyoxysilyl) propylamine coated slides stained with CD34 (Monoclonal, Mouse, Anti-human, Dako, clone QBEnd 10, Ready to use, Denmark, Product number-M716501-2) after antigen retrieval. Biotinylated anti-immunoglobulin was used as a secondary antibody for 30 minute. After being rinsed in Phosphate Buffered Saline (PBS), the slides were incubated with the peroxidase conjugated streptavidine label for 30 minutes at room temperature. The sections were again rinsed in PBS and incubated with diaminobenzidine for 7 minute. After chromogen development, slides were washed in two changes of water and counter stained with haematoxylin, washed in water, dehydrated, cleared and mounted. For positive control, oral squamous cell carcinoma tissue sections were used. For negative control, primary antibody was omitted from the section.
Immunohistochemistry Analysis
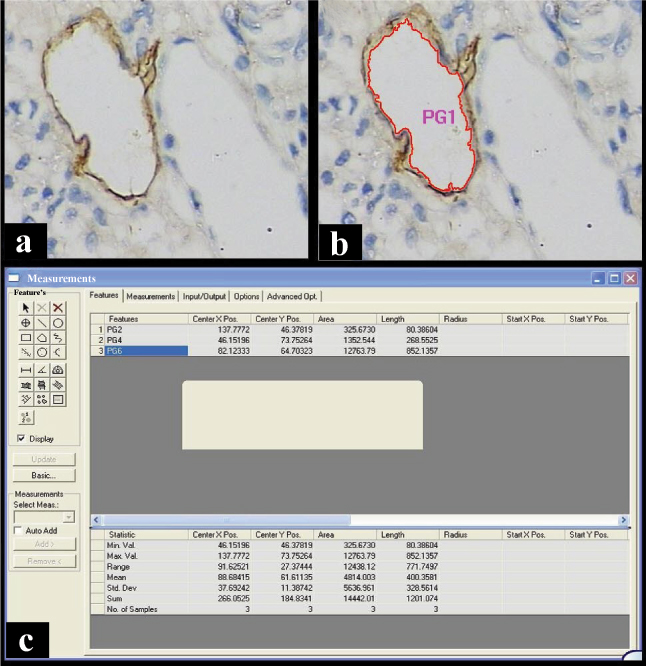
The generally acceptable criteria for a microvessel profile is an endothelial marker stained cell or cluster, that is separate from adjacent microvessel profiles and present within the tumor but not in necrotic and sclerotic zones [1,7]. Vessels with muscle wall were not counted. The assessment was carried out at the level of endothelial cells lining the blood vessels by their brown cytoplasmic staining [Table/Fig-1, 2]. Microvessel density was assessed at 400X magnification after initial scan at 100X magnification in areas showing the highest density of staining (hot spots). The field selection was performed by three senior oral pathologist to avoid subjectivity in selection criterion. In case of disagreements, consensus was obtained for selection of final area. Microvessels were counted by tracing them with the means of Computer assisted image analysis software Leica QWin V3 (Leica Microsystem – Leica DM 2500, Germany). Images were captured (400X magnification) in three most vascular fields next to the epithelium and quantified by means of computer assisted image analyses for MVD, TVA and MVA for CD34 positive slides. All microvessels were traced in one field and the number of vessels traced was considered as MVD, the total of areas of all traced vessels was considered as TVA and software gave value of MVA by dividing the TVA with MVD.
Photomicrograph showing CD34 positive endothelial cells in (a) Normal Oral mucosa(NOM), (b) Stage I oral submucous fibrosis; (c) Stage II oral submucous fibrosis; (d) Stage III oral submucous fibrosis. (Immunohistochemistry, Total Magnification 400X)
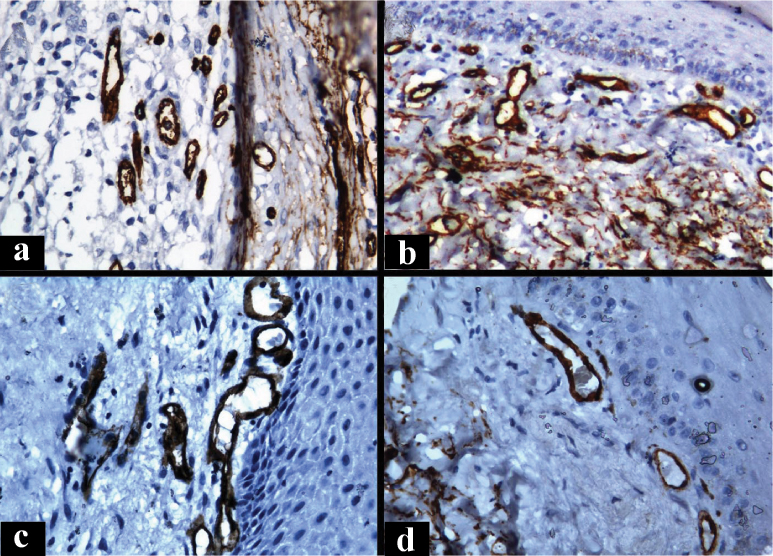
Image showing the (a) photomicrograph of CD34 Positive vessels, (b) in the same image the vessels traced with morphometric software and (c) the output window of morphometry software. (Immunohistochemistry, Total Magnification 400X)
Statistical Analysis
Statistical analysis was performed using SPSS 16.0 software for windows (SPSS, Inc, Chicago, IL, USA). The relationship between angiogenesis in various groups of OSMF and control was statistically analysed. Non parametric test ANOVA and Kruskal Wallis was used to compare between Stage I, II, III, IV OSMF and control group. A Mann-Whitney U test was used as a post hoc test to compare between the two groups. A p-values <0.05 were considered statistically significant.
Results
Demographic data
In OSMF group, age ranged from 16 to 66 years with a mean of 36.68±13.4. Predominantly males (34) were associated with OSMF than females (11) with a male to female ratio of 3.09:1. Mouth opening for OSMF patients ranged from 6 mm to 36 mm with a mean of 24.11±12.02. There were 15 cases in each stages of OSMF.
Immunohistochemistry
MVD was more in Stage I (17.02±4.72) OSMF followed by control (13.83±2.64), Stage II (12.80±2.61) and Stage III (9.52±1.28) with statistically significant differences (p<0.001). Furthermore, statistically significant differences were observed in the MVD between control versus Stage III OSMF (p=0.00). Similarly, TVA was statistically significant when compared between control versus OSMF (p<0.001), control versus Stage II OSMF (p=0.01), control Stage III OSMF (p<0.001), Stage I OSMF versus Stage II OSMF (p=0.05), Stage I OSMF versus Stage III OSMF (p<0.001), and Stage II OSMF versus Stage III OSMF (p<0.001). For MVA, statistically significant differences were between control versus OSMF (p=0.05), control versus Stage II OSMF (p<0.001), control versus Stage III OSMF (p<0.001), Stage I versus Stage III OSMF (p<0.001) and Stage II versus Stage III OSMF (p<0.001) [Table/Fig-3, 4].
Values of vascular parameters in Normal Oral Mucosa (NOM) and different stages of OSMF.
| Parameter | Study groups | Mean±SD | p value |
|---|
| Mean vascular density | Control | 13.83±2.64 | <0.001* |
| Stage I OSMF | 17.02±4.72 |
| Stage II OSMF | 12.80±2.61 |
| Stage III OSMF | 9.52±1.28 |
| Total vascular area | Control | 3579.13±1608.28 | <0.001* |
| Stage I OSMF | 2876.73±1381.36 |
| Stage II OSMF | 1996.57±886.37 |
| Stage III OSMF | 608.56±160.34 |
| Mean vascular area | Control | 251.82±98.94 | <0.001* |
| Stage I OSMF | 187.25±119.11 |
| Stage II OSMF | 160.00±75.76 |
| Stage III OSMF | 65.41±21.40 |
Statistically significant; p value obtained using ‘Kruskal Walllis test’.
Comparison of MVD, TVA and MVA in NOM and different stages of Oral Submucous Fibrosis.
| Mann-Whitney U test | MVD | TVA | MVA |
|---|
| NOM | 0.09 | 0.28 | 0.06 |
| OSMF Stage I |
| NOM | 0.30 | 0.01* | <0.001 |
| OSMF Stage II |
| NOM | <0.001* | <0.001* | <0.001* |
| OSMF Stage III |
| OSMF Stage I | 0.01* | 0.05* | 0.90 |
| OSMF Stage II |
| OSMF Stage I | <0.001* | <0.001* | <0.001* |
| OSMF Stage III |
| OSMF Stage II | <0.001* | <0.001* | <0.001* |
| OSMF Stage III |
Statistically significant; p value obtained using ‘Mann-Whitney U test’. NOM- Normal oral mucosa
Discussion
OSMF is an insidious, chronic, irreversible progressive disease in which the symptoms vary from burning sensation of oral mucosa to inability in opening mouth, difficulty in swallowing and speech [8]. In the early stage, epithelial hyperplasia and mild fibrosis may be seen and later stage shows atrophy of epithelium and hyalinization of connective tissue. Tissue remodeling process is dependent on blood supply of the tissue. Angiogenesis is seen when there is increased requirement of nutrition [9].
Neoangiogenesis is a highly organized multistep process in which steps like vessel sprouting, endothelial cell migration, proliferation, tube formation and survival are involved [10]. Although angiogenesis cannot be measured directly, it can be inferred by quantification of vasculature, usually in the form of MVD [11], thus providing an index of angiogenesis [1].
In the present study, MVD increased in early stage, decreased in advanced stages and TVA and MVA decreased from normal oral mucosa to increasing stages of OSMF. This indicates that there is only increase in the number of vessels in early stage of OSMF which decreases in later stages and the vessels size and area goes on decreasing as the severity of fibrosis increases.
Our findings were in accordance with Fang CY et al., who also observed increase in MVD in early stages while decrease in MVD in later stages of OSMF [12]. They stated microvessel hyperplasia occurring in early stages of OSMF leads to increase in MVD.
Rajendran R et al., observed similar values of MVD in various stages of OSMF which were marginally low when compared to normal oral mucosa. They observed increase in Mean Vascular Area Percentage (MVAP) and Mean Vascular Luminal Diameter (MVLD) with progression of OSMF [4]. It is believed that stromal alteration is seen in OSMF and it progresses with the stage of OSMF [4].
This stromal alteration in the form of fibrosis, hypoxia promotes neovascularization. However, usual tissue reaction resultant to ischemia/hypoxia does not seem to operate in OSMF which is preconditioned by stromal changes as part of the disease process.
Desai SR et al. studied MVD, MVLD, MVAP and epithelial thickness (ET) in normal oral mucosa, different stages of OSMF and found statistically insignificant results with marginal rise in vasculature from normal to earlier stages OSMF and decline in advanced stages [1].
Desai SR et al., supported and Rajendran R et al., hypothesized that progressive deposition and cross-linkage of mature collagen bundles leads to hyalinization of stroma and it suffers resultant ischemia/ hypoxia because of physical and biochemical effects of the process [1,4]. As an adaptive response of pathologic mechanism, tissue tries to cope up with hypoxia by actively promoting neovascularization. Desai SR et al., further elaborated it as hypoxia activates a hypoxia inducible factor leading to transcription of vascular endothelial growth factor mRNA with resultant neoangiogenesis. Moreover, they stated that in OSMF stromal alteration is always present and is progressive as the disease advances. The possible role of the inducible nitric oxide synthetase, basic fibroblast growth factor, transforming growth factor- β, platelet derived growth factor and hypoxia inducible factor κ all of which are non endogenous angiogenic promoters, may be playing an important role in maintaining the vascularity of underlying connective tissue in OSMF [1].
As per the findings of the present study, it does not appear true that epithelial atrophy in OSMF is due to lack of perfusion, caused by decreased vascularity of subjacent connective tissue stroma [4]. The carcinogens from areca nut could accumulate either on or immediately below the epithelium to act for long duration before diffusion into deeper tissues. Super adding to this, less vascularity may hold back the quick absorption of carcinogens into the systemic circulation [4].
Singh M et al., observed that MVD was inversely proportional to stage of OSMF. MVLD and MVA increased in early stages and decreased in advanced stages of OSMF [13]. However, Sabarinath et al., found characteristic rise in MVD in all stages of OSMF than normal oral mucosa [10]. Amongst OSMF stages, marginal but statistically insignificant increase was found.
Debnath S et al., found increase in MVD in early stages and decrease in advanced stages of OSMF [14]. They also found that MVLD and MVAP increased with increasing stage of the OSMF. In two different studies Rooban T et al., and Kirankumar K et al., found no correlation between of stage of OSMF and extent of trismus [15,16]. As concluded by some authors that trismus occurs due to increase in subepithelial collagen deposition is an oversimplification [17,18].
Murgod VN et al., found increase in MVD in early stages and decrease in advanced stages of OSMF and exponentially rises in oral squamous cell carcinoma [19]. This is due to neoangiogenesis in the mucosa to compensate for the hypoxia created by fibrosis. The reduction of MVD in the advanced Stage Is attributed to more hyalinized stroma, diminished mediators of angiogenesis. And rise in MVD and MVA in oral squamous cell carcinoma is due to angiogenic switch triggered by increased demand for blood to provide nutrition for the proliferating tumour cells [19]. Increased MVD rather than vascular dilatation in early OSMF validates the old concept that progressive fibrosis compresses blood vessels and causes a reduction of luminal diameter with disease progression [20].
Garg N and Mehrotra R found no statistically significant difference in epithelial thickness, MVD, MVA, MVLD and luminal perimeter from normal oral mucosa to OSMF [20]. Pandiar D and Shameena PM found reduction in MVD from normal oral mucosa to OSMF in increasing stages and then it increased in oral squamous cell carcinoma [21].
We support the view that an altered cytokine activity, decreased vascularity and increased fibrosis creates atmosphere for carcinogens derived from tobacco and areca nut to act on already compromised epithelium [2]. Invasion and dysplasia of epithelial cells create angiogenic microenvironment by release of angiogenic factors leading to neoangiogenesis in turn causing increase in MVD as OSMF transforms into oral squamous cell carcinoma.
Incongruous results seen in previous studies of vasculature in OSMF may be due to differences in the classification system, sample size in each group, marker used for vascular endothelium and the parameters to be evaluated in vasculature [Table/Fig-5].
Details of studies done by different authors on vasculature in OSMF.
| Author | Classification | Stain/ Marker used for vascular endothe- lium | Cases in study groups | Comparison with NOM / OSCC | Cases in Stage I | Cases in stage II | Cases in stage III | Cases in stage IV | Parameters studied | Results |
|---|
| Fang CY et al (2002) (12) | NA | NA | 27 | NA | NA | NA | NA | NA | MVD, MVA, MVAP | MVD increased in early OSMF. MVD, MVA and MVAP reduced in middle and late stages of OSMF. |
| Rajendran R et al (2005)(4) | Rajendran R et al 2003 | H&E stain | 20 | NOM-10 | 10 | 10 | - | - | MVD, MVAP, MVLD | MVD was same in control and study group. MVAP and MVLD increased with increasing stage of OSMF. |
| Singh M et al (2010)(13) | Pindborg and Sirsat | H&E stain, Van Gieson’s stain | 83 | No | 9 | 32 | 39 | 3 | Koilocytes (no/lpf), Collagen thickness (μm), Endothelial cells (no/lpf), MVA, MVLD | Koilocytes and collagen thickness increased with increasing stage of OSMF. Endothelial cell decreased with increasing stage of OSMF. MVA and MVLD increased in Stage II than Stage I and reduced in Stage III and Stage IV. |
| Desai SR et al (2010)(1) | Lai DR et al | CD34 | 30 | NOM-10 | 0 | 4 | 2 | 17 | ET, MVD, MVLD, MVAP, | ET reduced from normal to Stage II, Stage IV to Stage III. MVD, MVLD, MVAP increased in Stage II and reduced in Stage III and Stage IV |
| Sabarinath B et al (2011) (10) | Pindborg and Sirsat. modified into 3 stages VEOSMF, EOSMF and MAOSMF | Anti factor VIII, Von Willibrand Factor | 30 | NOM-10 | 9 | 14 | 7 | - | MVD, MCD | MVD increased than NOM in all stages of OSMF. Positive correlation was seen between MVD and MCD |
| Debnath S et al (2013)(14) | Pindborg and Sirsat | H&E stain, Van Gieson’s stain, Masson’s Trichrome stain, Acid Fuschin stain | 100 | No | 36 | 29 | 28 | 7 | ET, Subepithelial collagen thickness, No. of endothelial cells/s. μm MVA, MVLD, Compared vascular parameters with trismus | ET showed no statistical difference. MVD, MVA, MVLD decreased with increasing stage of OSMF. No correlation was found with trismus. |
| Murgod VN et al (2014) (19) | Pindborg and Sirsat. modified into 2 stages EOSMF and AOSMF | H&E stain | 60 | NOM-30 WDSCC- 30 | 30 | 30 | - | - | MVD, MVA, MVAP, MVLD | MVD, MVA, MVLD increased from NOM to OSMF to OSCC. All parameters increased in early OSMF and reduced in advanced OSMF and again increased in OSCC. |
| Pandiar D and Shameena PM (2014) (21) | Pindborg and Sirsat | CD34 bFGF | 30 | NOM-10, OSMF-D -5 OSMF-M -2 cases | 00 | 11 | 17 | 2 | ET, Comparison between CD 34 and bFGF positive vessels MVD | CD34 is a better marker of vascular endothelium than bFGF. ET and MVD decreased with increasing stage of OSMF than NOM and MVD increased exponentially in OSCC |
| Garg N and Mehrotra R (2014)(20) | Pindborg and Sirsat | H&E stain | 35 | NOM-10 | 7 | 14 | 9 | 5 | ET, MVA, MVLD, Vessel perimeter | ET reduced from NOM to increasing stages of OSMF. MVA, MVLD and vessel perimeter in OSMF was less than NOM, all parameters reduced Stage II, Stage I, Stage III and Stage IV sequentially |
| Present study (2017) | Modification of Lai DR et al into 3 stages; I, II and III | CD34 | 45 | NOM-15 | 15 | 15 | 15 | - | MVD, MVA, TVA | MVD increased in Stage I than NOM and reduced in Stage II and Stage III OSMF. MVA and TVA reduced from NOM to Stage I, Stage II Stage III OSMF sequentially. |
Lpf- Low power field, ET- Epithelial thickness, MCD- Mast cell density, NA- Not available, VEOSMF – Very early OSMF, EOSMF - Early OSMF, MAOSMF – Moderately advanced OSMF, AOSMF - Advanced OSMF NOM-Normal oral mucosa
The criteria for including cases for vasculature evaluation should be clinical, preferably inert-incisal mouth opening, so that subjectivity in different observer can be minimized. Classification stating status of vessels causes a bias in sample selection. Sample size in each group should be sufficient to get statistically significant results and comparison with normal oral mucosa should be done. Many markers have been tried for staining blood vessels and CD34 has come out to be a better marker than other markers like CD31, basic fibroblast growth factor, H&E stain and specials stains [10,13,14,19-21]. It also excludes the bias of including lymphatic vessels in counting. Different authors have used many parameters to study vasculature in health and disease like MVD, TVA, MVA, MVLD, MVAP and vessel perimeter. Amongst these MVD, TVA and MVA/MVAP don’t change when there is change in vessel from dilated to compressed. But parameters like MLVD and vessels perimeter do not give clear idea about the vessel being dilated or compressed and the values vary with change in shape. The results of this study need to be confirmed by long term follow up of patients with OSMF and its transformation into oral squamous cell carcinoma.
Limitation
The morphometric analysis of vessels is a two dimensional study of three dimensional microvasculature. It can only detect the morphological state of the vasculature and not the functional state. In vivo studies on three dimensional functional state of vasculature need to be planned by using advanced techniques.
Conclusion
The present study supports previous findings that vascularity increases progressively from normal mucosa to OSMF. The increase in vascularity in the early stages could be due to inflammation and a compensatory attempt of the mucosa to deal with ischemia. Further, there is no neoangiogenesis in advanced stages of OSMF and with progressive fibrosis; the vascularity is reduced, which may predispose the tissue to atrophic changes in the overlying epithelium and subsequent malignant changes. Guidelines need to be decided for parameters to be studied in future vasculature studies in OSMF to make them more analogous.
Lpf- Low power field, ET- Epithelial thickness, MCD- Mast cell density, NA- Not available, VEOSMF – Very early OSMF, EOSMF - Early OSMF, MAOSMF – Moderately advanced OSMF, AOSMF - Advanced OSMF NOM-Normal oral mucosa