Gliomas are the most common brain tumour with an annual incidence of 5-10/100,000 persons. Among gliomas, the most common histology is GBM [1]. Mean age of presentation of glioma is 40-45 years and that of GBM is 55-60 years. Patients diagnosed with anaplastic astrocytoma have a median survival of three years after diagnosis but the median survival is only 14 months following the diagnosis of GBM [1].
The treatment of GBM has evolved over the years from surgery alone to surgery followed by postoperative Radiotherapy (RT). Studies have shown that extent of resection has prognostic importance. Pichlmeier U et al., reported that patients without residual contrast-enhancing tumour had a higher overall median survival time than those with residual enhancing tumour (17.9 vs 12.9 months, respectively (p<0.001) [2].
Randomized trials have demonstrated a clear survival benefit of the use of RT after surgery [3]. In a systemic review of postoperative RT in GBM, Laperriere N et al., showed significant (p<0.001) survival benefit of postoperative RT over supportive care [4]. Relative risk of GBM patients treated with postoperative RT for one-year mortality was 0.81. The understanding of tumour biology and recurrence patterns helped to shift from whole brain RT practice to partial brain irradiation.
In a phase III trial comparing concurrent chemoradiation with TMZ and adjuvant six cycles TMZ 150-200 mg/m2 every four weeks, Stupp R et al., showed increased median OS of 14.6 months compared to 12.1 months in the RT only arm and two year survival of 26.5% compared to 10.6% in the RT only arm [5]. Now, the standard of care for a newly diagnosed patient of GBM is maximal safe resection followed by concurrent chemoradiation with TMZ and then further adjuvant TMZ for six cycles as per regimen by Stupp R et al., and referred to as conventional TMZ (C-TMZ) further in this article [5].
Several modifications are being tried in order to further improve the survival over that conferred by C-TMZ [5]. With a hypothesis that continuous daily administration of TMZ is more effective than a single dose, clinical trials with TMZ explored a wide range of dosing schedules aiming at maximum O6-Methylguanine Methyltransferase (MGMT) depletion in tumour cells [6]. However, this concept failed to show any improvement in the RTOG 0525 trial [7] which randomly assigned patients with newly diagnosed GBM to receive standard radiation and six cycle’s maintenance TMZ or dose dense TMZ for 12 cycles.
Another strategy postulated is to use extended cycles of adjuvant TMZ (E-TMZ) beyond the current standard of six cycles as used in C-TMZ regimen. Studies have shown promising survival with the use of E-TMZ; however, the reports are mostly limited to retrospective studies with significant bias [8,9].
We did this prospective randomized study with a hypothesis that E-TMZ for total of 12 adjuvant cycles would improve OS of patients with newly diagnosed GBM as compared to C-TMZ. We also sought to assess the difference in the toxicity profiles of these two regimens.
Materials and Methods
Patient Characteristics
Between January 2012 and July 2013, 40 postoperative cases (within eight weeks of surgery) with confirmed histopathology of GBM were recruited prospectively at the Department of Radiation Oncology, All India Institute of Medical Sciences, New Delhi, India. Eligible patients had to be between the age of 18-65 years with Karnofsky Performance Score (KPS) ≥70, haemoglobin ≥10 g/dL, leukocyte count ≥ 3000/mm3, absolute neutrophil count ≥ 1500/ mm3, platelets ≥ 100,000/ mm3, creatinine clearance ≥ 50 mL/min, and normal liver function tests. Patients with significant co-morbid conditions (which could preclude use of TMZ), those with recurrent or metastatic disease or synchronous/metachronous malignancy were excluded from the study. All patients signed informed consent before entry into the study and the study was approved by Institutional Ethics Committee (IESC/T-027/2011).
Study Design
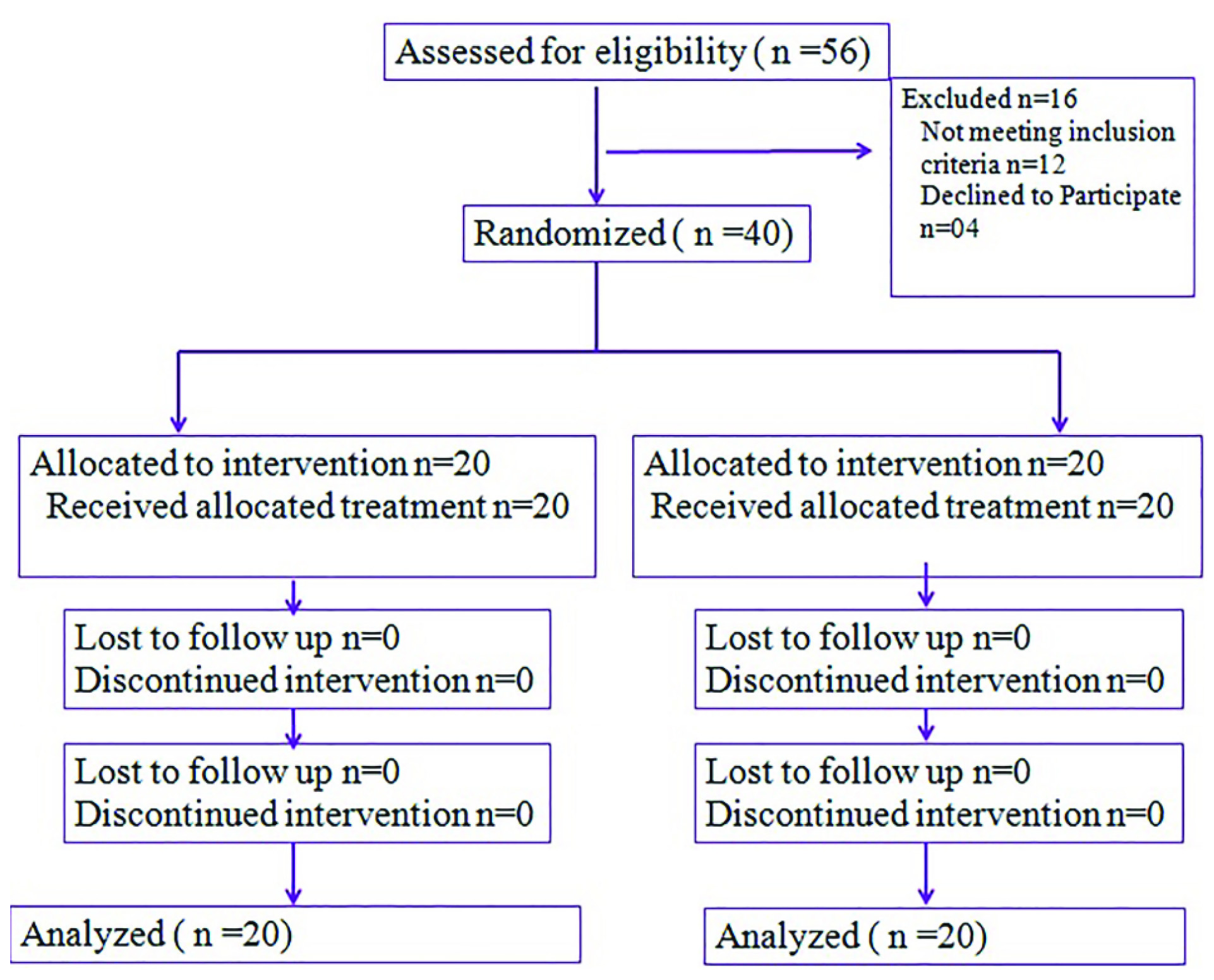
The present study was a non-blinded, prospective, parallel randomized trial. Forty eligible patients were randomized as per computer generated random numbers in to one of the two treatment arms: C-TMZ or E-TMZ. Patients in both the treatment arms underwent maximum safe surgical resection {gross total excision (> 90% resection), sub-total excision (< 90% resection) or decompression only} and received similar regimen of concurrent chemoradiotherapy. Subsequently, patients in C-TMZ arm and E-TMZ arm respectively received adjuvant TMZ to a maximum of 6 or 12 cycles respectively. A flow diagram of the progress of trial is summarized in [Table/Fig-1].
Flow diagram of the trial
Treatment
RT was planned in a phased manner. For the initial phase, Clinical Target Volume (CTV) was generated by a 2 cm isotropic margin to preoperative lesion as evident on T2 FLAIR Magnetic Resonance Imaging (MRI) scan with further editing (from bone and normal uninvolved structures). Second phase radiation volume encompassed the preoperative T1 contrast enhanced changes with a margin of 2 cm. Planning Target Volume (PTV) was generated by giving 5 mm isotropic expansion to respective first phase and second phase CTV. Fifty gray to the initial PTV followed by 10 gray to second phase PTV were delivered on a linear accelerator with a three dimensional conformal plan.
Concurrent chemotherapy (TMZ 75 mg/m2 daily) and adjuvant chemotherapy with TMZ 150 mg/m2 (for the first cycle) and 200 mg/m2 (for the subsequent cycles, if well tolerated) were used and adjuvant chemotherapy was repeated every four weeks.
For salvage therapy, re-surgical excision was tried in eligible patients. Bevacizumab (10 mg/kg intravenous day one) and irinotecan (125 mg/m2 intravenous day one) every two weeks was given and in patients found unsuitable for this, metronomic TMZ (100 mg/ m2 from day 1-21 repeated every four weeks) was used. Salvage chemotherapy was used in patients till progression or limited by general condition of the patient or toxicities.
Toxicity Evaluation and Follow up
During RT, patients were sampled for complete blood counts every week. Prior to initiation of each adjuvant TMZ cycles, patients were sampled for complete blood count as well as liver and kidney function tests. Patients were evaluated for toxicities using CTCAE version 3.0 [10]. At each follow up, a detailed clinical and neurological assessment was done. For response assessment, contrast enhanced MRI of brain was done after three months of completion of RT course and this was repeated every three months thereafter. On clinical suspicion of progression, MRI was ordered earlier than the routine three months of interval. Response evaluation was done by Macdonald DR et al., Criteria [11].
Statistical Analysis
Survival was calculated from the time of diagnosis. Progression Free Survival (PFS) was calculated from the time of diagnosis to the time of progression or death. OS was calculated from the time of diagnosis to the time of death from any cause. Kaplan Meier method [12] was used for survival analysis. A sample size was not directly calculated for the study. The sample size was limited to 40 on the basis of available resources. A p-value of <0.05 was taken as significant and SPSS version 12.0 (SPSS Inc., Chicago, IL, USA) was used for all statistical analysis.
Results
Patient characteristics are summarized in [Table/Fig-2].
| Characteristics | C-TMZ arm | E-TMZ arm |
|---|
| Median age [(yrs) (range)] | 49 (19-65) | 44 (19-62) |
| Sex ratio [male: female] | 10:10 | 14:06 |
| Median KPS at presentation (range) | 90 (70-100) | 80 (70-100) |
| Maximum tumour diameter (cm) (range) | 4.8 (2.3-8.0) | 5.5 (2.1-8.0) |
| mMedian symptom duration {(months) (range)} | 1.5 (0.5-24) | 2 (1-20) |
Abbreviations: C-TMZ= Conventional temozolomide arm, E-TMZ=Extended temozolomide arm, KPS= Karnofsky performance status
Overall, two arms combined, most common presentation was with symptoms of raised intracranial tension (70%) followed by seizures (20%). Frontal lobe location was most common (50%), followed by temporal lobe (25%), parietal lobe (10%) and others (15%).
Treatment and Toxicity
Gross total resection was possible in 12 (60%) and 11 (55%) patients and subtotal resection was done in 8 (40%) and 9 (45%) patients respectively in C-TMZ and E-TMZ arm. Median interval time between diagnosis and surgery was one month in both the arms.
Median time between surgery and initiation of RT was 39 days (range, 21-69 days) in C-TMZ arm and 40 days (range, 23-90 days) in E-TMZ arm. All patients included in the study received complete course of RT (60 gray in 30 fractions over a period of six weeks) except one in E-TMZ arm (radiation was interrupted for 10 days after 46 gray due to Grade 4 neutropenia). All patients received concurrent TMZ during RT. Grade 1 nausea was observed in 18 patients in both the arms. Highest frequency of Grade 2 non-haematological toxicity was seen in 10 (50%) and 9 (45%) patients respectively in C-TMZ and E-TMZ arm and no patient suffered from Grade 3 non haematological toxicities non-haematological toxicities in both the arms have been summarized in [Table/Fig-3].
Summarizes Grade 2 non-hematological toxicities in two treatment arms during concurrent chemoradiotherapy
| Toxicity* | C-TMZ arm Number of patients (%) | E-TMZ arm Number of patients (%) |
|---|
| Anorexia | 2 (10) | 2 (10) |
| Fatigue | 10 (50) | 9 (45) |
| Headache | 3 (15) | 2 (10) |
| Nausea | 4 (20) | 3 (15) |
| Vomiting | 2 (10) | 3 (15) |
| Insomnia | 1 (5) | 2 (10) |
Abbreviations: C-TMZ= Conventional temozolomide arm, E-TMZ=Extended temozolomide arm
Toxicity may add to more than 50% in C-TMZ arm and 45% in E-TMZ arm as patients in each arm had multiple toxicities.
Two patients had grade 2 haematological toxicity in both C-TMZ (both thrombocytopenia) and E-TMZ arm (one Grade 4 neutropenia and one thrombocytopenia) during concurrent RT. Overall, 0% and 5% respectively in C-TMZ and E-TMZ arm had haematological toxicity ≥ 3 in grade during concurrent phase.
Median number of adjuvant TMZ cycles was six (range, three to six) and 12 (range, 3-12) in C-TMZ and E-TMZ arm respectively. During adjuvant chemotherapy, one patient in each arm had Grade 3 and one patient in E-TMZ arm had Grade 4 thrombocytopenia. One patient in E-TMZ arm also had Grade 3 neutropenia. One patient in each arm had Grade 2 anaemia. Overall, 5% and 15% patients respectively in C-TMZ and E-TMZ arm had haematological toxicity ≥3 in grade.
Survival Outcomes
Median follow up duration for the entire cohort was 17.25 months (range, 5.31-36.03 months). Median follow up in C-TMZ and E-TMZ arm were 14.65 months (7.54-32.75 months) and 19.85 (5.31-36.03 months) respectively. Forteen patients in each arm had progression of disease. Median PFS was 12.8 months and 16.8 months in C-TMZ and E-TMZ arm respectively.
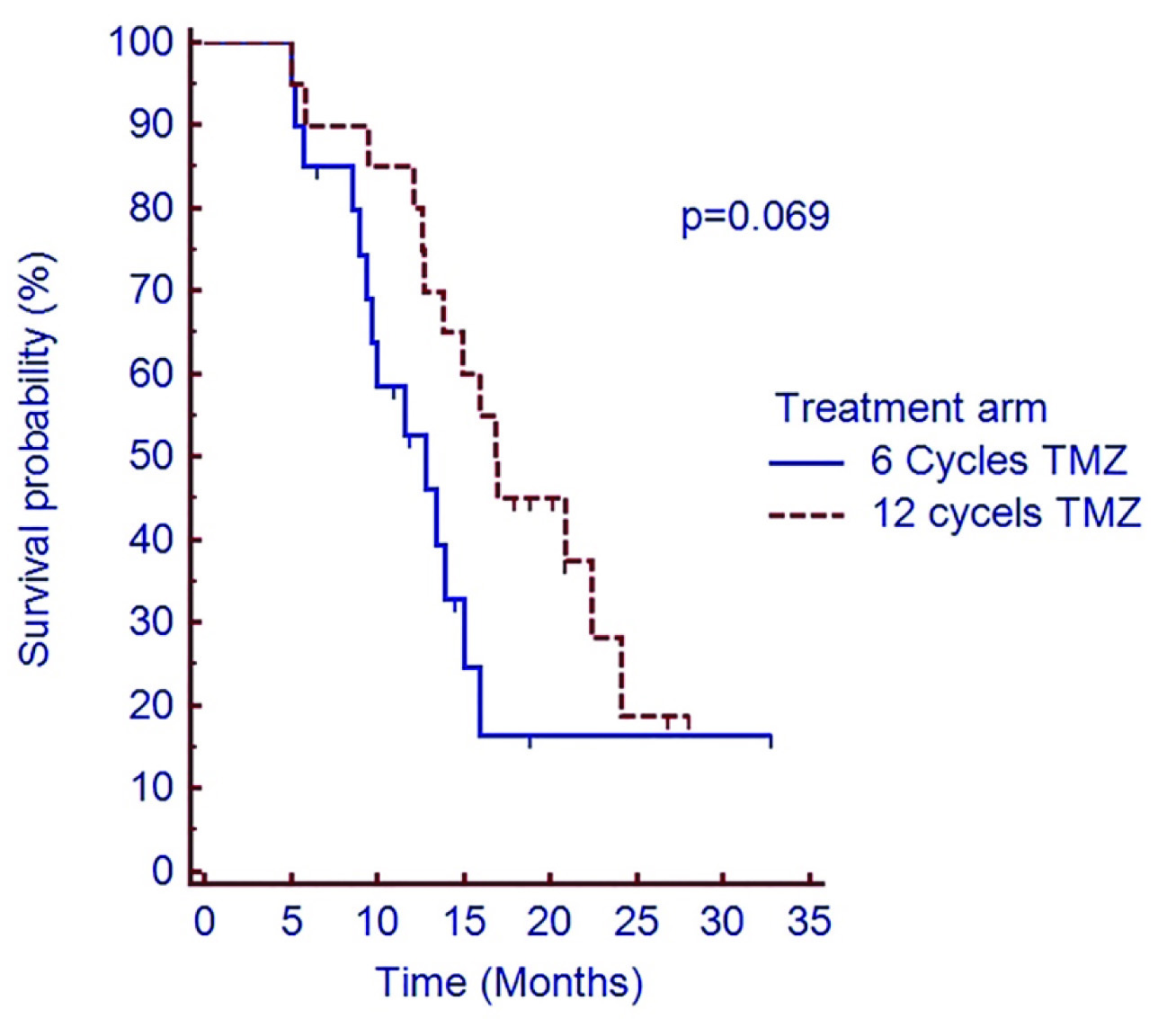
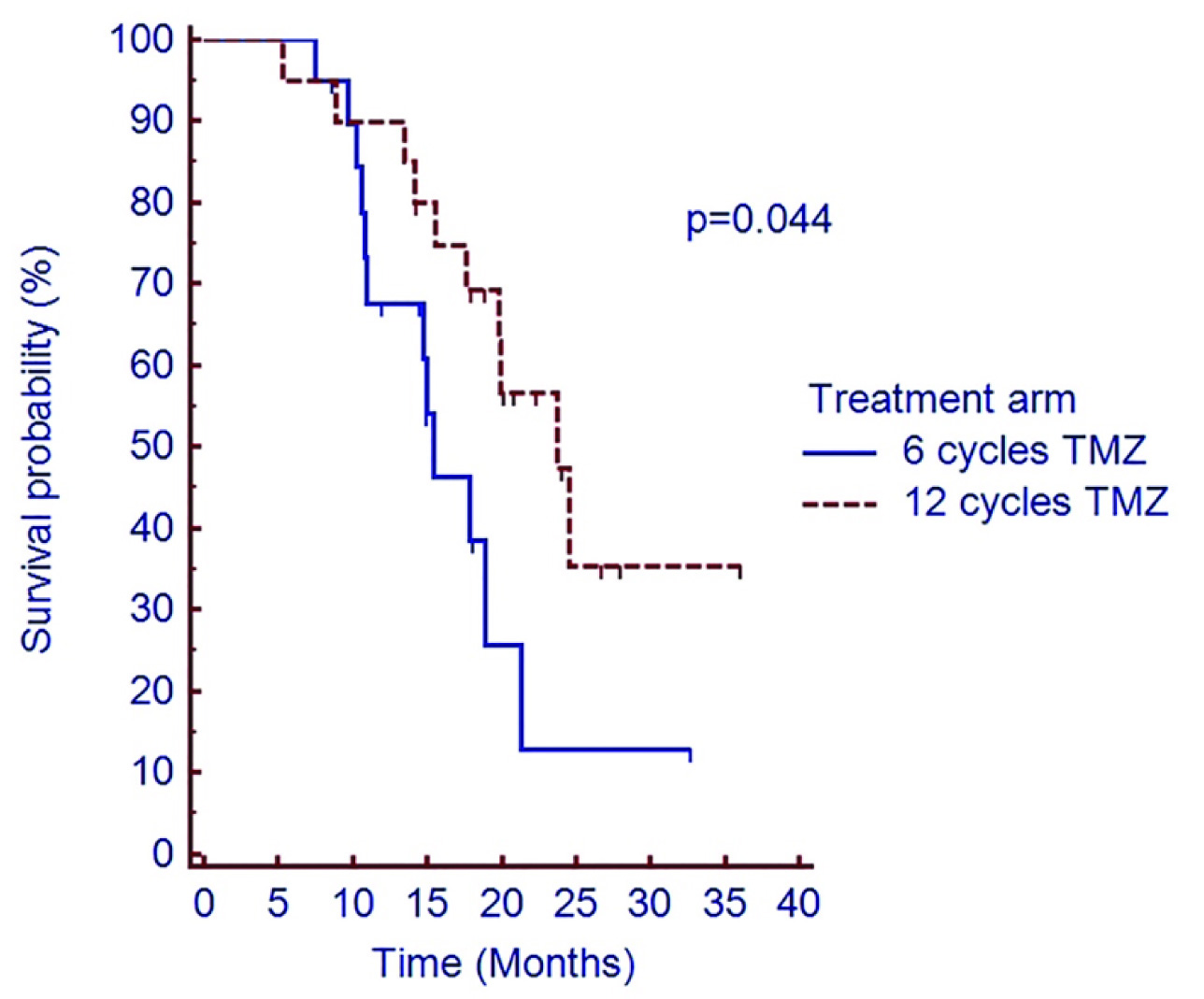
Two year PFS was 16.4% vs. 18.7% in C-TMZ and E-TMZ arm respectively (p=0.069) [Table/Fig-4]. Median OS was 15.4 months vs. 23.8 months in C-TMZ and E-TMZ arm respectively. Two year OS was 12.9% vs. 35.5% respectively in C-TMZ and E-TMZ arm (p=0.044) [Table/Fig-5].
Shows PFS of patients treated with adjuvant temozolomide in C-TMZ and E-TMZ arms
Abbreviation: PFS=Progression free survival, C-TMZ= conventional six cycles temozolomide, E-TMZ= 12 cycles of extended temozolomide
Shows overall survival of patients treated with adjuvant temozolomide in C- TMZ and E-TMZ arms
Abbreviations: OS=Overall survival, C-TMZ= conventional six cycles temozolomide, E-TMZ= 12 cycles of extended temozolomide
Patterns of Failure and Salvage Therapy
Of 14 patients in C-TMZ arm who had progression; 10 had local recurrence, three had recurrence in a different lobe and one had local as well as recurrence elsewhere in brain. Two patients underwent re-surgical excision of the recurrent tumour, two patients received palliative chemotherapy with bevacizumab and irinotecan (median of three cycles, range three to six), two patients received metronomic temozolomide and eight patients received only best supportive care. Four patients had partial response to the salvage therapy, while two progressed on therapy.
Of 14 patients in E-TMZ arm who had progression; eight had local recurrence, 4 had recurrence in a different lobe and two had both local as well as recurrence elsewhere in brain. Three patient underwent re-surgery (two of these also received postoperative radiotherapy, 45 Gray in 25 fractions over five weeks), four patients received metronomic TMZ, three patients received bevacizumab and irinotecan (median six cycles, range three to six) and four patients were found suitable for only best supportive care. Two patients had complete response, four had partial response and four had progressive disease to salvage therapy.
Discussion
The current standard of care after maximum safe resection for GBM is concurrent TMZ and RT followed by six cycles of adjuvant monthly TMZ. Although improved from before, the outcomes with this protocol remains dismal. Approximately 70% of patient progress during one year and only approximately 27% are alive at two years [5]. There is always a reluctance to shift from the established treatment regimen for any disease and same applies to GBM too. However, recently there has been an effort to modify this protocol in order to optimize the treatment outcome and improve survival.
The proven efficacy of TMZ in the adjuvant management of GBM prompted researchers to find out if increasing the cumulative dose of TMZ might improve outcome. This could be achieved either by increasing the dose of TMZ in each cycle (hence intensifying the dose schedule) or by extending the duration of conventionally used schedule of adjuvant TMZ. The concept behind these modifications is increasing the exposure of TMZ causing sustained depletion of MGMT [6]. RTOG 0525 tested this concept and randomized patients to convention six cycles of TMZ versus 12 cycles of dose dense schedule (75-100 mg/m2 of TMZ given for 21 days of a month) [7]. However, neither median OS (16.6 vs. 14.9 months; p = 0.63) nor median PFS (5.5 vs. 6.7 months; p =0.06) was improved and this was also not different by MGMT methylation status. Grade ≥3 toxicity was significantly higher in the dose dense arm (53% versus 34%; p<0.001).
Sun H et al., found in a meta-analysis that the intensified regimens (like 50 mg/m2, day 1-28; 150 mg/m2, day 1-7 and then day 15-21; 100 mg/m2, day 1-21) did not reveal any OS or PFS advantage (HR 1.07, 95% CI 0.94-1.22; p=0.31) as compared to regimens with higher peak concentration during a short period of time (daily doses ≥ 150 mg/m2/day within ≤7 days/cycle) [13]. The intensified regimens also predispose patients to higher rates of leukopenia. The results of these studies suggest that intensified approach of delivering TMZ might not be the way forward to achieve superior clinical outcome and alternative methods like extended duration of conventional TMZ schedule may be worth exploring.
[Table/Fig-6] summarizes the results of current studies focusing on the use of E-TMZ. The present study is an update of our previous published result [14]. Median OS for patients with E-TMZ regimen in these studies [8,9,15-18] have ranged from 24 to 31 months as compared to patients with conventional number of TMZ cycles (range: 8 to 16.5 months). In the only prospective phase II study, Refae AA et al., randomized 59 patients to six cycles of adjuvant TMZ (n=29) or >6 cycles of adjuvant TMZ (N=30). Both PFS and OS were statistically better in the arm receiving extended duration of TMZ [Table/Fig-6] [18].
Summarizes contemporary literature on the use of extended duration of TMZ.
| Authors/Year of study | Type of study | Patients with GBM | Number of Adjuvant TMZ cycles | Survival outcome |
|---|
| Hau P et al.,/ 2007[15] | Retrospective multi-institutional [50 German Centers]; Patients receiving at least 12 cycles or 12 months of TMZ were included in this study. | 57% (n=73) | Median 13 (9-40) | Median OS 30.6 months (95% CI= 22.4 to 37.4 months) for GBM patients (n= 35) |
| Barbagallo GMV et al., /2014 [16] | Retrospective comparative analysis of patients receiving >6 cycles (Group A) and up to 6 cycles of TMZ (Group B) | n=19 (Group A) n=18 (Group B) | Mean 27±26 vs. 4±2 | Median OS 28 vs. 8 months Median PFS 20 vs. 4 months (Group A vs. B) |
| Seiz M et al.,/2010 [17] | Retrospective comparative analysis of patients receiving >6 cycles (Group A) and up to 6 cycles of TMZ (Group B) | n=59 (Group A) n=55 (Group B) | Median 6 (6-57) | Median OS 15 months and 2-year OS 27.5% for entire cohort. Number of cycles (p<0.001) correlated with overall survival as well as time to progression (p < 0.0001, HR = 0.911) |
| Malkoun N et al.,/ 2012[9] Roldán Urgoiti GB et al/ 2012 [8] | Retrospective single institutional analysis Retrospective comparative analysis of patients receiving >6 cycles (Group A) and 6 cycles of TMZ (Group B) | n=46 n=29 (Group A) n=23 (Group B) | Median 6 (0-34) Median 6 cycles | Median 2-year OS 1.7% and PFS 10.4% Median survival of 16.5 months in Group B and 24.6 months in Group A (p=0.031) |
| Refae AA et al.,/2015 [18] | Prospective phase II study Arm 1 (6 cycles of TMZ) Arm 2 (>6 cycles of TMZ) | n=29 (Arm 1) n=30 (Arm 2) | Median 11 cycles (range 8-23) | Median PFS 12.1 months (Arm 1) versus 18.8 months (Arm 2); p=0.015 Median OS18.1 months (Arm 1) versus 24.1 months (Arm 2); p=0.048 |
| Present study | Prospective randomized study of patients receiving 6 (C-TMZ) versus 12 (E-TMZ) | n=20 (C-TMZ) n=20 (E-TMZ) | Median 6 cycles (C-TMZ) Median 12 cycles (E-TMZ) | Median OS was 15.4 months vs. 23.8 months in C-TMZ and E-TMZ arm respectively (p=0.044) |
Abbreviations: TMZ=Temozolomide; C-TMZ=Conventional temozolomide; E-TMZ=Extended temozolomide; GBM=Glioblastoma multiforme; OS= Overall survival; PFS=Progression free survival
In the present study, the median OS was 23.8 months in E-TMZ arm as compared to 15.4 months in C-TMZ arm and this is in concurrence with other reported studies [Table/Fig-6]. Extended duration of TMZ has been found to be well tolerated in these studies and rates of Grade III or higher haematological toxicity has remained <10% [15]. Additionally, it has been seen that with increasing number of cycles, stoppage due to side effects also becomes less common [17]. In our study, 0% and 5% had haematological toxicity ≥ 3 in grade during concurrent phase and 5% and 15% patients had haematological toxicity ≥3 in grade during adjuvant phase in C-TMZ and E-TMZ arms respectively.
One significant issue with premature discontinuation of adjuvant TMZ has been noted to be because of pseudoprogression. This has been seen in as much as 50% of cases [19]. This was not accounted for in the study by Stupp R et al., and this may have impacted use of TMZ and consequently survival of patients [5]. Pseudoprogression should be taken in to account while following up patients during the adjuvant phase of treatment and in absence of true clinical and radiological progression of the disease, TMZ should be continued and based on the results of the studies, this should be more than six cycles.
The maximum duration for which extended TMZ can be used is still not clear. Studies support use up to eight years [20] without any serious side effects. Mannas JP et al., reported use of up to 85 cycles of TMZ in their cohort of five patients of malignant glioma treated with E-TMZ [21]. It appears that the tolerability and safety does not depend on the cumulative doses of TMZ cycles and in patients who tolerate it well for initial 6-12 cycles continue to tolerate it well. However, the reported long term use has been in patients with different histologies of glioma and this should be kept in mind while extrapolating this use to GBM patients.
Limitation
One limitation of our study is the small sample size of patients and lack of information on MGMT methylation status of patients. MGMT hyper-methylation has been shown to correlate favorably with outcome of the GBM patients and also predict better outcome with the use of TMZ. However, in the seminal study by Stupp R et al., even patients with un-methylated MGMT benefited from the use of TMZ. Hence, the results of our study could also be applied to patients of GBM irrespective of their MGMT status [5]. Having said that, we do acknowledge that the information on MGMT status would have added more value to the results of our study and we strongly recommend it in future studies.
Conclusion
The result of our study suggests that extended duration of adjuvant TMZ is safe, tolerable as well as confers significant survival advantage as compared to conventional duration of TMZ. Pending results from larger, phase III, multi-institutional studies, it appears prudent for us to suggest use of at least 12 cycles of adjuvant TMZ in patients with freshly diagnosed GBM.
Abbreviations: C-TMZ= Conventional temozolomide arm, E-TMZ=Extended temozolomide arm, KPS= Karnofsky performance status
Abbreviations: TMZ=Temozolomide; C-TMZ=Conventional temozolomide; E-TMZ=Extended temozolomide; GBM=Glioblastoma multiforme; OS= Overall survival; PFS=Progression free survival