Mucoepidermoid Carcinoma of Salivary Gland: Limitations and Pitfalls on FNA
Geetha Vasudevan1, Arijit Bishnu2, Brij Mohan Kumar Singh3, Varun Kumar Singh4
1 Additional Professor, Department of Pathology, Kasturba Medical College, Manipal University, Manipal, Karnataka, India.
2 Assistant Professor, Department of Pathology, Melaka Manipal Medical College, Manipal University, Manipal, Karnataka, India.
3 Assistant Professor, Department of Immunohematology and Blood Transfusion, Kasturba Medical College, Manipal University, Manipal, Karnataka, India.
4 Junior Resident, Department of Pathology, Kasturba Medical College, Manipal University, Manipal, Karnataka, India.
NAME, ADDRESS, E-MAIL ID OF THE CORRESPONDING AUTHOR: Dr. Arijit Bishnu, Department of Pathology, Melaka Manipal Medical College, Manipal University, Madhavnagar, Manipal-576104, Karnataka, India.
E-mail: arijit.bishnu@manipal.edu
Mucoepidermoid Carcinoma (MEC) is the most common malignant neoplasm of salivary gland origin. However, its morphologic heterogeneity poses difficulty in interpretation. In the present series we discuss the morphologic features of MEC, limitations and pitfalls in its diagnosis on Fine Needle Aspiration Cytology (FNAC). Fourteen cases of suspected MEC were evaluated cytologically followed by histopathological examination for confirmation. A definite cytological diagnosis was rendered in nine cases; three of the remaining five were underdiagnosed as abscess, pleomorphic adenoma and mucus cyst. Of the remaining two cases, one case each of sebaceous carcinoma and sialadenitis was mislabeled as MEC on cytology. A satisfactory aspirate composed of intermediate cells, mucin secreting cells and squamous cells in a mucinous background may not be obtained in all cases of low grade MEC. High grade MEC can be classified as squamous cell carcinoma. Hence, awareness of confounding factors with clinicopathologic correlation and judicious use of frozen section can help in minimizing errors.
Discordance, Low grade, Sebaceous carcinoma, Sialadenitis, Under-diagnosis
Introduction
FNAC is an important and accurate diagnostic tool in the pre-operative evaluations of salivary gland lesions [1,2]. However, contradiction remains regarding its role in making type specific diagnosis due to overlapping features and morphologic variability amongst tumours [1].
Case Series
A total of 14 cases of FNAC were evaluated over a period of four years in a tertiary care hospital. Cytology and histopathology records were reviewed during this period and after recording relevant clinical details, cytologic and histopathologic discordance was identified in five of the 14 cases.
Cytologic features evaluated were clusters of epithelial cells, mucoid background, intermediate cells, mucin secreting cells and squamous cells. The diagnosis of MEC was based on the presence of the above mentioned features in varying proportions.
In the present series 12 of 14 reviewed cases were histologically confirmed as MEC. Of these, five were low grade, four were intermediate grade and three were high grade tumours. The age incidence ranged from 17-76 years with a male preponderance and male to female ratio being 3:1. Parotid was the most common site involved (10 cases). Palate and submandibular gland were affected in one case each.
The morphologic features in the FNA smears were very heterogeneous. Smears showed variable cellularity with fragments of intermediate cells with round to oval nuclei, moderate amount of dense cytoplasm along with mucus cells in a background of muciphages, mucus and nuclear debris in the low grade tumours. The high grade variants were cellular, with poorly cohesive clusters and singly scattered squamous cells with moderate to abundant eosinophilic cytoplasm, pleomorphic hyperchromatic nuclei, and mitosis in a necrotic background and resembled squamous cell carcinoma.
Of these 14 cases 9 were diagnosed correctly on cytology. The false negative and false positive cases are shown in [Table/Fig-1]. In our series, three cases of MEC were underdiagnosed as pleomorphic adenoma, mucus cyst and abscess on cytology. The two cases of false positive cases included one each of sebaceous carcinoma and chronic sialadenitis [Table/Fig-1].
CasCases with discordant cytologic and histopathologic feature (n=5).
| SL. No | Patient age/sex | Site | Cytological diagnosis | Histopathological diagnosis | Cytological features |
|---|
| 1. | 57 years/female | Submandibular gland | Pleomorphic adenoma | MEC (intermediate grade) | Clusters of epithelial cells, plasmacytoid and spindle shaped, myoepithelial cells and mesenchymal cells, chondromyxoid background and histiocytes |
| 2. | 59 years/male | Parotid | Mucus cyst | MEC (low grade) | Occasional benign acinar cells, ductal epithelial cells, foamy macrophages, lymphocytes, neutrophils, stromal fragments and mucinous background |
| 3. | 47 years/male | Palate | Abscess | MEC (low grade) | Paucicellular, neutrophils, histiocytes and karyorrhectic debris, mucicarmine+ |
| 4. | 55 years/male | Parotid | Low grade MEC | Chronic sialadenitis with cystic duct dilatation with foci of squamous and mucus cell metaplasia | Few large cells with round nuclei and abundant vacuolated cytoplasm suggestive of mucus cells, mucinous background, lymphocytes and small cluster of ductal epithelial cell |
| 5. | 72 years/emale | Parotid | High grade MEC | Sebaceous carcinoma | Clusters of malignant squamoid cells, high N:C, moderate cytoplasm, hyperchromatic nuclei, background of acinar cells, foamy macrophages, cellular debris, neutrophils, lymphocytes, giant cells and mucus |
Discussion
MEC is a common malignant neoplasm of the salivary gland but at times leads to diagnostic difficulty on cytology. Identification of mucus cells, intermediate cells and squamous cells in smears are necessary for definitive diagnosis [3]. Ironically, not all these features are present conspicuously in all cases, especially the low grade lesions [1,2]. In such a scenario, MEC may be mistaken for other benign or malignant entities. In the present series, nine cases were accurately diagnosed as MEC on cytology. The false positive and the false negatives included three cases of low grade variants of MEC. The cases were considered false positives when an FNAC diagnosis of MEC was suggested and diagnosed as other entity on histopathology.
One case of low grade MEC, was mistaken for mucus cyst on FNAC [Table/Fig-2]. Careful review of the FNA slides did not reveal any cytologic element suggestive of low grade MEC. Paucicellularity due to sampling error or cyst fluid diluting the tumour cells, presence of occasional mucus cells, mucinous background and lymphocytes can contribute to this underdiagnosis. Low grade MEC are often cystic [4,5], and may pose diagnostic confusion with mucus cyst and other benign cystic lesions [1,3]. Khafaji et al., have described Warthin tumours and lymphoepithelial cysts causing diagnostic difficulty with MEC particularly of the low grade variant on FNA, owing to the bland cytological features, hypocellularity or non-representative nature of the aspirate [2].
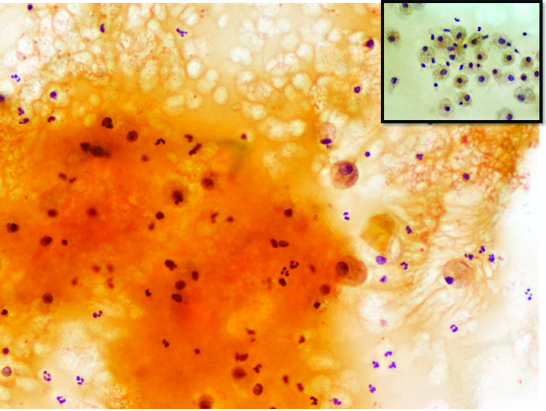
FNAC revealed abundant mucinous material containing aggregates of muciphages and mixed inflammatory infiltrate (Papanicolaou 20X). Inset showing the mucus cells resembling muciphages (Papanicolaou 40X). This was interpreted as consistent with mucus cyst.
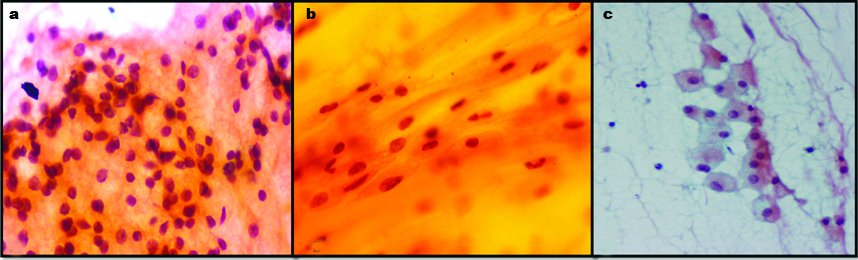
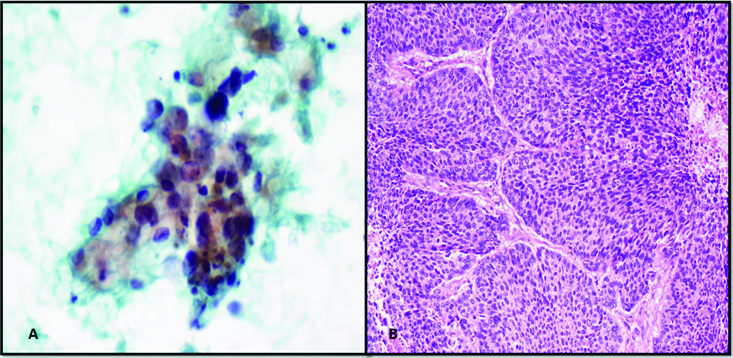
In our series, one case of MEC was mislabeled as pleomorphic adenoma. Underdiagnosis as pleomorphic adenoma is a recognized pitfall as observed by Kotwal M et al., [6]. The intermediate cells and stromal cells were misinterpreted benign epithelial cells and myoepithelial cells of pleomorphic adenoma respectively [Table/Fig-3]. Furthermore, thick extracellular mucin was perceived as pale chondromyxoid matrix. In addition, the mucus cells were interpreted histiocytes [Table/Fig-3]. Various authors have commented on these pitfalls [6-8]. The misdiagnosis of MEC as pleomorphic adenoma has also been described by Joseph TP et al., and Kocjan G et al., they opined that the presence of squamous metaplasia can lead to this error [1,9].
a) Intermediate cells in low grade MEC misinterpreted as epithelial cell (Papanicolaou 40X); b) Misinterpretation of mucus and intermediate cells in mucinous background as cells entrapped in chondromyxoid material (Papanicolaou 40X); c. Mucus cell mistaken for histiocytes in low grade MEC (Papanicolaou 20X). (All Images from left to right)
A third case of low grade MEC was diagnosed as abscess on cytology. Paucicellularity of the smear, failure to demonstrate squamoid and mucus cells and predominance of neutrophils, histiocytes and karyorrhectic debris contributed to the misdiagnosis. This error can perhaps be overcome by reaspiration from the tissue adjacent to the cystic area. Young JA et al., recognized leukocytes, cell debris and degenerate epithelial cells owing to secondary infection or presence of lymphoid rich infiltrate causing mistaken diagnosis [10]. MEC, acinic cell carcinoma and warthin tumours are few of the common salivary gland tumours known to have a rim of dense lymphoplasmacytic infiltrate [4,5]. Selective sampling from these areas during FNAC may contribute to diagnostic errors.
Chronic sialadenitis can also be mistaken for low grade MEC on cytology. In our series, a 55-year-old male patient with chronic obstructive sialadenitis had cystically dilated duct filled with inspissated mucin. In addition, the ductal lining epithelium showed foci of mucus and squamous metaplasia [Table/Fig-4,5]. Aspiration of these metaplastic cells in a background of inspissated mucus lead to a suspicion of low grade MEC [Table/Fig-6]. Many workers have discussed these pitfalls in obstructive duct lesions which can pose problems because of mucous and squamous metaplasia [3-11]. Judicious utilization of frozen section, as was requested by the cytologist in the current case is a valuable tool to minimize errors in such scenario.
Small areas of the specimen contained the characteristic histology of MEC in the case diagnosed as Pleomorphic Adenoma on FNAC (H&E 10X; inset H&E 40X).
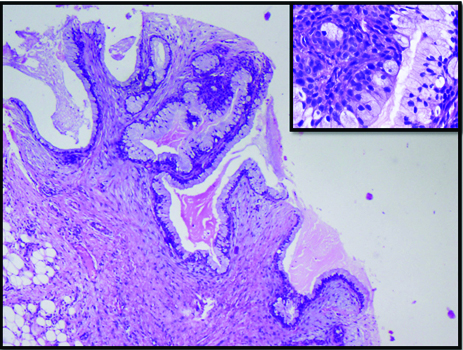
Dilated duct with inspissated mucin and duct wall showing mucus cell and squamous cell metaplasia in chronic sialadenitis. (H&E 20X; inset H&E 4X).
a,b) Paucicellular smear showing cells with round nuclei and vacuolated cytoplasm suggestive of mucus cells (arrow head) in a wispy mucinous background with small cluster of ductal epithelial cell (Papanicolaou 20X). Low grade MEC could not be excluded and a frozen section was advised.
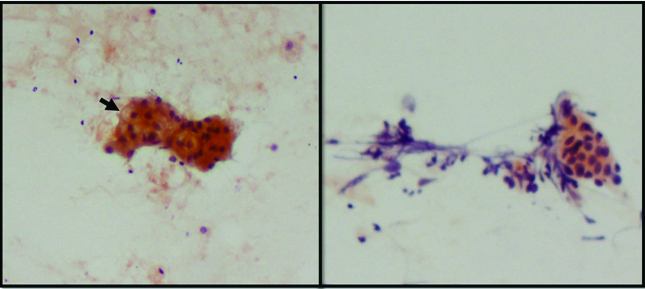
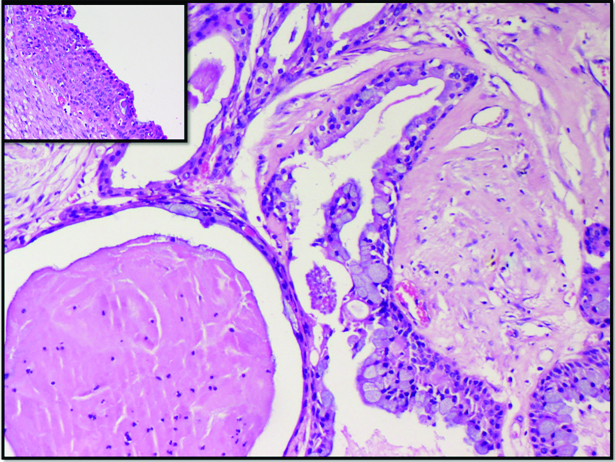
A metastatic sebaceous carcinoma in the region of the parotid was diagnosed as high grade MEC on FNAC. The cytologic diagnosis was based on the presence of clusters of malignant squamoid cells in a background of few foamy macrophages and giant cells [Table/Fig-7]. In this case, lack of clinical history (i.e., the patient being a known case of sebaceous carcinoma of the eyelid) and the rarity of sebaceous carcinoma presenting as a parotid mass contributed to an error in sub-classification of malignant process. However, the high grade tumour morphology was accurately identified.
a) Malignant squamoid cells in clusters and scattered singly overdiagnosed as high grade MEC (Papanicolaou 40X); b) Metastatic sebaceous carcinoma (H&E 20X).
Conclusion
FNAC is an important tool in the evaluation of salivary gland lesions. A satisfactory aspirate composed of intermediate cells, mucin secreting cells and squamous cells is necessary to diagnose low grade MEC but may not be always available. High grade MEC can mimic squamous cell carcinoma. Timely communication with the clinician while dealing with paucicellular aspirates where a possibility of low grade MEC is considered needs emphasis. Hence, awareness of confounding factors with clinico-pathologic correlation, proper sampling with judicious use of frozen section can help in minimizing errors.
[1]. Joseph TP, Joseph CP, Jayalakshmy PS, Poothiode U, Diagnostic challenges in cytology of mucoepidermoid carcinoma: Report of 6 cases with histopathological correlationJ Cytol 2015 32:21-24. [Google Scholar]
[2]. Al-Khafaji BM, Nestok BR, Katz RL, Fine-needle aspiration of 154 parotid masses with histologic correlation-Ten-year experience at the University of Texas M. D. Anderson Cancer CenterCancer Cytopathology 1998 84:153-59. [Google Scholar]
[3]. Rupani AB, Kavishwar VS, Achinmane V, Puranik GV, Fine needle aspiration cytology of low-grade mucoepidermoid carcinoma of the parotid gland: A diagnostic challengeJ Cytol 2008 25:115-16. [Google Scholar]
[4]. Veder LL, Kerrebijn JD, Smedts FM, den Bakker MA, Diagnostic accuracy of FNAC in warthin tumoursHead Neck 2010 32:1635-39. [Google Scholar]
[5]. Bocatto P, Altavilla G, Blandamura S, Fine needle aspiration biopsy of salivary gland lesions-a reappraisal of pitfalls and problemsActa Cytol 1998 42:888-98. [Google Scholar]
[6]. Kotwal M, Gaikwad S, Patil R, Munshi M, Bobhate S, FNAC of salivary gland - A useful tool in preoperative diagnosis or a cytopathogist’s riddle?J Cytol 2007 24:85-88. [Google Scholar]
[7]. Orell SR, Sterrett GF, Whitaker D, Klijanienko J, Head and neck; salivary glands. In: Orell SR, Sterrett GF, Whitaker D, editorsFine Needle Aspiration Cytology 2005 5th edEdinburghChurchill Livingstone-Elsevier:49-71. [Google Scholar]
[8]. Elhosseiny A, Salivary gland. In:Koss LGKoss’ Diagnostic Cytology and its Histopathologic basis 2006 Volume 25th edLippincott Williams & Wilkins:1229-1261. [Google Scholar]
[9]. Kocjan G, Nayagun M, Harris M, Fine neddle aspiration cytology of salivary gland lesions: Advantages and pitfallsCytopathology 1990 1:269-65. [Google Scholar]
[10]. Young JA, Diagnostic problems in fine needle aspiration cytopathology of the salivary glandsJ Clin Pathol 1994 47:193-98. [Google Scholar]
[11]. Mukunyadzi P, Review of fine needle aspiraition cytology of salivary gland neoplasms with emphasis on differential diagnosisAm J Clin Pathol 2002 118:S100-15. [Google Scholar]