Salvage Surgery for Metastatic Gall Bladder Cancer with Vanishing Liver Metastasis Following Palliative 5-Fluorouracil Metronomic Chemotherapy
RDR Somasekar1, Obla Naganathbabu2, Raju Prabhakaran3, Murugaiyan Gnanasekar4, Devy Gounder Kannan5
1 Postgraduate, Department of Surgical Gastroenterology, Institute of Surgical Gastroenterology, Madras Medical College, Chennai, Tamil Nadu, India.
2 Director, Department of Surgical Gastroenterology, Institute of Surgical Gastroenterology, Madras Medical College, Chennai, Tamil Nadu, India.
3 Assistant Professor, Department of Surgical Gastroenterology, Institute of Surgical Gastroenterology, Madras Medical College, Chennai, Tamil Nadu, India.
4 Assistant Professor, Department of Surgical Gastroenterology, Institute of Surgical Gastroenterology, Madras Medical College, Chennai, Tamil Nadu, India.
5 Former Director, Department of Surgical Gastroenterology, Institute of Surgical Gastroenterology, Madras Medical College, Chennai, Tamil Nadu, India.
NAME, ADDRESS, E-MAIL ID OF THE CORRESPONDING AUTHOR: Dr. Obla Naganathbabu, Institute of Surgical Gastroenterology, Madras Medical College, Chennai- 600003, Tamil Nadu, India.
E-mail: naganathbabu@gmail.com
Gall Bladder Cancer (GBC) is an aggressive malignancy. The prognosis of metastatic GBC is dismal. Until recently, there are no standard treatment guidelines for management of patients with metastatic GBC. But the ultimate aim of any treatment modality is to improve the overall survival with good quality of life. We hereby report a long term survivor of metastatic GBC treated with initial six cycles of gemcitabine + cisplatin combination chemotherapy, but did not show any response. In view of chemotoxicity and as a matter of physician preference patient was started on low dose weekly 5- Fluorouracil monotherapy based on a metronomic chemotherapy principle. There was complete resolution of all the liver metastasis and salvage radical cholecystectomy was done. The patient is disease free on imaging at eight months of follow up. A very few such cases have been reported in the world literature, till date.
Metastatic disease must not be considered incurable as there are no objective measures to predict tumour biology. Further research on metronomic chemotherapy and tumour genetics in such patients will open a new era of individualised cancer therapy based on objective measures that will predict tumour biology.
Tumour biology, Biliary tract cancer, Cholecystectomy, Low dose chemotherapy
Case Report
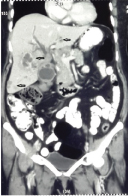
A 48-year-old female with a BMI of 26 kg/m2 had symptoms of right upper quadrant pain for one month. She had no jaundice, anorexia or weight loss. On clinical examination there were no positive findings other than hepatomegaly. An ultrasound examination of the abdomen revealed hepatomegaly with multiple ill defined lesions in both lobes of the liver with inhomogeneous echoes. The gall bladder was distended with diffuse irregular asymmetrical wall thickening and there were no gall stones or sludge. Her blood biochemistry reports were essentially normal. A triple phase Contrast-Enhanced CT abdomen (CECT) confirmed the diagnosis of carcinoma gall bladder with multiple bilobar liver metastases [Table/Fig-1]. In view of the Stage IVb disease, an ultrasound guided biopsy of the liver lesion was done which revealed metastatic adenocarcinomatous deposit. The CA 19-9 level was 565 U/ml. Her performance status was Eastern Cooperative Oncology Group score 1 (ECOG 1). She was planned for palliative Gemcitabine + Cisplatin combination chemotherapy.
Multidetector Computed Tomography (MDCT) abdomen showing multiple liver metastasis (arrows) in both sides, on a coronal multiplanar reconstruction image.
The chemotherapy schedule she received during each cycle is as follows:
Cisplatin 25 mg/m2 of body surface area followed by gemcitabine 1 gm/m2, each administered intravenously on days 1 and 8 as a three weekly cycle. She completed six cycles. A response evaluation with triple phase CECT abdomen at the end of 18 weeks revealed no significant response of both the primary tumour and the liver metastasis and the CA 19-9 level was 561 U/ml. But she developed grade 2 (National Cancer Institute, Common Terminology of adverse events Version 4.0) cisplatin induced peripheral neuropathy and grade 2 anaemia.
As the performance status of the patient continued to be good with no deterioration, she was started on weekly 5- Fluorouracil 350 mg/m2 (total dose of 500 mg intravenously) monotherapy as second line palliative chemotherapy, purely as a matter of physician preference. After six cycles the CA 19-9 level dropped to 245 U/ml though CECT abdomen was unremarkable. After the 12th cycle, there was a significant response with disappearance of few of the liver lesions. At the end of 18 cycles there was complete resolution of all the liver metastasis with partial regression of the primary tumour on an MRI abdomen.
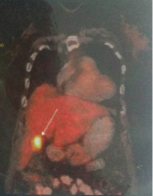
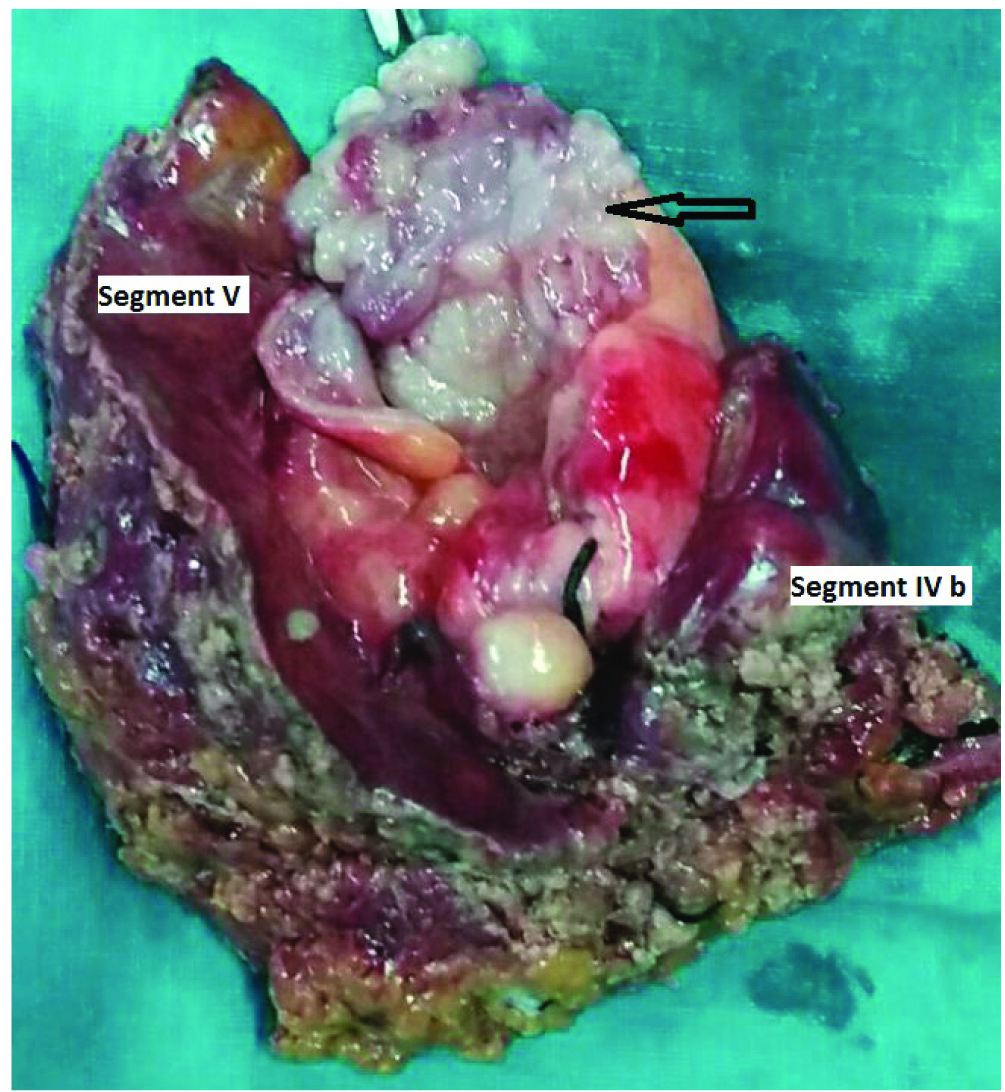
A whole body PET-CT revealed no PET activity in the liver and elsewhere [Table/Fig-2]. An intensely FDG avid (Standard Uptake Value SUVmax 13.1) soft tissue density mass lesion involving the fundus of the gall bladder was noted. A radical cholecystectomy with removal of segments IVb and V of the liver [Table/Fig-3] was done. Patient developed ISGLS (International Study group of Liver Surgery) Grade A post hepatectomy bile leak which settled with conservative management.
Whole body PET scan showing an Fludeoxyglucose (18F) (FDG) avid foci in the region of gall bladder (arrow) with no FDG avid foci elsewhere.
Radical cholecystectomy specimen with cut open gall bladder with the proliferative lesion (arrow) along with liver segments IV b and V.
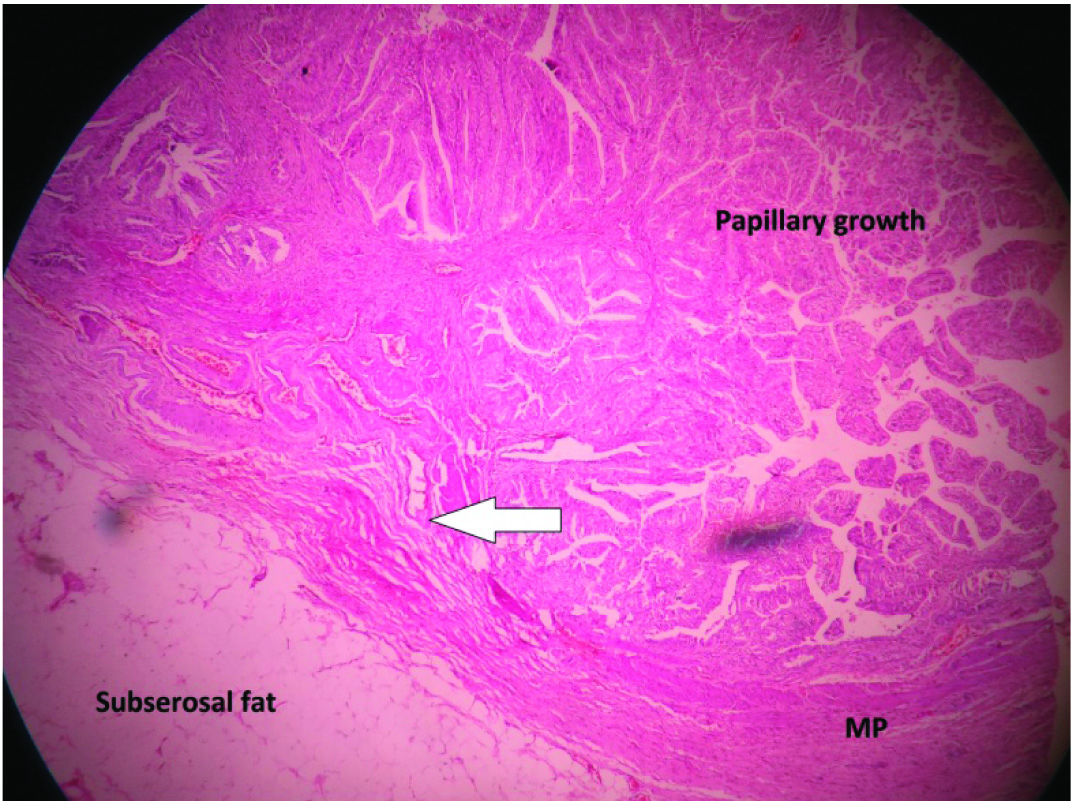
Histopathology examination revealed an infiltrating well differentiated papillary adenocarcinoma infiltrating into the muscle layer pT1b [Table/Fig-4], cystic duct margin was free of tumour, adjacent liver showed fatty changes and three out of three lymph nodes showed reactive hyperplasia. She was given six cycles of adjuvant weekly 5-FU and now at the end of eight months following surgery she continues to be disease free on a triple phase CECT abdomen.
HPE slide 10X view showing the layers of the gall bladder wall with a papillary growth infiltrating (arrow) into the muscularis propria (MP) of the gall bladder wall.
Discussion
GBC is the most common and the most aggressive of all the biliary tract cancers. The median survival of patients with metastatic disease is 5.8 months [1]. Our case is an exception with the patient surviving for more than 18 months from diagnosis. As there are no objective measures to predict tumour biology till date, patients with locally advanced or metastatic disease must not be deemed incurable. The performance status of the patient at initial presentation and over the course of a planned treatment is possibly a good indicator of the tumour biology.
The beneficial role of chemotherapy as compared to best supportive care for advanced GBC has been proved beyond doubt in randomised controlled trials [2]. The ABC 02 trial has proved the superiority of gemcitabine+cisplatin combination chemotherapy to gemcitabine alone for advanced biliary tract cancers [3]. In our case, though the disease was stable, patient developed anaemia and peripheral neuropathy due to chemotherapy.
In an attempt to decrease chemotherapy induced toxicity, to maintain tumour dormancy and to prolong patient survival, it was decided to start weekly 5-Fluorouracil monotherapy at a low dose as metronomic chemotherapy. Metronomic chemotherapy is an emerging and evolving methodology. The basic principle of metronomic chemotherapy is administering anti-cancer drugs in low doses continuously or at regular intervals but more often than the cyclical conventional anti-cancer therapy schedules [4]. Metronomic chemotherapy exerts its action by inhibiting tumour angiogenesis, inducing antitumour immunity and tumour dormancy [5]. There are no standard metronomic chemotherapy regimens till date. In our case, the initial fall in CA 19-9 level was encouraging, patient’s tolerance was good and so metronomic chemotherapy was continued. Similarly as the patient has responded well to 5-FU we decided to continue the same postoperatively. As there is no standard guideline regarding adjuvant chemotherapy in GBC and this being an unusual presentation, it was decided to stop postoperative weekly 5-FU monotherapy after six cycles and observe the patient.
In our case, we speculate that the probable reason for 5-FU sensitivity might be low expression of Dihydropyrimidine dehydrogenase [6] (an enzyme which degrades 5-FU) or Thymidylate synthase (principal target enzyme of 5-FU) in the metastatic clone of tumour cells in the liver. High thymidylate synthase activity in tumour tissue lowers the response to 5-FU [6]. An increased OPRT (Orotate Phosphoribosyl Transferase) activity in tumour cells increases the efficacy of 5-FU [6].
Newer DNA microarray technology can predict resistance or response to chemotherapeutic drugs [7]. To the best of our knowledge only nine cases of advanced GBC treated with curative surgery following chemotherapy have been reported [6,8,9] and ours is possibly the tenth case. For these nine cases, gemcitabine alone, S1 alone or gemcitabine+S1 were used. Our thorough search of the available medical literature has also shown that there are no reports on the use of metronomic chemotherapy in GBC and ours is possibly the first.
Conclusion
Patients with metastatic disease must not be considered incurable as there are no objective measures to predict tumour biology. Metronomic chemotherapy can be tried in selected cases of GBC especially when they cannot tolerate standard conventional chemotherapeutic regimens due to toxicity. Further research on tumour genetics and metronomic chemotherapy in such patients will open a new era of individualised cancer therapy. Geneticist will become part of the multidisciplinary team for cancer care in the years to come.
[1]. Duffy A, Capanu M, Abou-Alfa G, Huitzil D, Jarnagin W, Fong Y, Gallbladder cancer (GBC): 10-year experience at Memorial Sloan-Kettering Cancer Centre (MSKCC)Journal of Surgical Oncology 2008 98(7):485-89. [Google Scholar]
[2]. Sharma A, Dwary A, Mohanti B, Deo S, Pal S, Sreenivas V, Best supportive care compared with chemotherapy for unresectable gall bladder cancer: a randomized controlled studyJournal of Clinical Oncology 2010 28(30):4581-86. [Google Scholar]
[3]. Valle J, Wasan H, Palmer D, Cunningham D, Anthoney A, Maraveyas A, Cisplatin plus gemcitabine versus gemcitabine for biliary tract cancerNew England Journal of Medicine 2010 362(14):1273-81. [Google Scholar]
[4]. Muthusamy P, Chary KV, Nalini GK, Metronomic chemotherapy: seems prowess to battle against cancer in current scenarioJournal of Clinical and Diagnostic Research 2016 10(11):FC09-FC13. [Google Scholar]
[5]. Maiti R, Metronomic chemotherapyJournal of Pharmacology and Pharmacotherapeutics 2014 5(3):186 [Google Scholar]
[6]. Kitajima K, Kobayashi S, Shiba H, Uwagawa T, Ishida Y, Aiba K, Successful treatment of advanced gallbladder cancer with an anticancer drug S-1: assessment based on intratumoral geneInternational Journal of Clinical Oncology 2008 13(6):545-51. [Google Scholar]
[7]. Trevino V, Falciani F, Barrera-Saldaña HA, DNA microarrays: a powerful genomic tool for biomedical and clinical researchMolecular Medicine 2007 13(9-10):1 [Google Scholar]
[8]. Okumura T, Nakamura J, Kai K, Ide Y, Nakamura H, Koga H, Curative resection of gallbladder cancer with liver invasion and hepatic metastasis after chemotherapy with gemcitabine plus S-1: report of a caseWorld Journal of Surgical Oncology 2014 12(1):326 [Google Scholar]
[9]. Morimoto H, Ajiki T, Takase S, Fujita T, Matsumoto T, Mita Y, Resection of gallbladder cancer with hepatic metastasis after chemotherapy with gemcitabineJournal of Hepato-Biliary-Pancreatic Surgery 2008 15(6):655-58. [Google Scholar]