The number of adults with diabetes in developing countries is predicted to be increased by 69% between 2010 and 2030 alongside urbanization and 75% of them is deliberated to be from developing countries by 2025, with the principal statistics from, India and China [1]. The majority of patients with Type 2 diabetes in India are underweight in comparison to the high occurrence of obesity in the same group in the developed world (80%-90%). What is elusive in these observations is whether the low body weights head up the onset of diabetes, or occur as a repercussion of it [2,3]? The hypothesis that undernourishment refuges a populace from diabetes was improperly developed on indirect evidence accessible from primary epidemiologic studies [4]. Traditional concept of symbiosis between obesity and diabetes risk developed during world wars is challenged by increased prevalence in developing realms [5].
With the augmented occurrence of diabetes mellitus among the malnourished domains, initial cataloguing by WHO (1985) as “malnutrition related diabetes mellitus was refurbished by a new terminology “malnutrition modulated diabetes mellitus”. In this scenario, a study by Gupta SK et al., exhibited that amongst urban Indians, rate of diabetes was 3.6% and 1.5% in normal and lean subjects respectively [6]. The epidemiologic study of diabetes and malnutrition is confounded with inappropriate data supports as in countries where malnutrition level is high proper data structure of Health Survey Research (HSR) is lacking.
Nationwide study done in 1979 on the incidence of diabetes in India, involving 35,000 volunteers from all divisions of the population contemplated the absence of incongruity in the diabetes prevalence in rural and urban populations (1.9% and 2.4%, correspondingly) [7,8]. On the contrary, surveys by Rao KSJ et al., in a habitually undernourished rural population in India inferred that the majority people troubled by diabetes mellitus belonged to traditionally underprivileged category who consumed inadequate diets and is likely to have resulted in metabolic derangement involving diabetes melliutes [9]. It was speculated that continued protein deprivation may be a crucial stimulator of diabetes, either by gradually decreasing beta cell function in conjunction with islet cell mass and increasing the predisposition to genetic or environmental diabetogenic stimuli like the immunological destruction, dietary toxins and certain viral infections [3,5]. According to the “something inherited, something added” hypothesis regarding the pathogenesis of diabetes, proposed by Cerasi E and Luft R, under nutrition may initiate overt diabetes in a hereditarily susceptible individual [10]. Deviance from the usual comes out to be abhorrent with a noticeable connection between the opposite limits of the nutritional scale in the kinship to diabetes.
Increased occurrence of the illness which was previously accredited as “a disease of riches” amongst the poor sectors, together with health complications burden the economy of developing realms [11]. These findings procured from epidemiological studies project the prevalence of “diabetes epidemic” even if obesity levels remain persistent [12]. In the background of the above debate, the elevated prevalence rate of diabetes amongst the undernourished populaces necessitates wide ranging illustrations to cross-examine the overwhelming position of obesity as the most significant environmental causative factor of diabetes mellitus, since the time of Joslin (1921) [13]. Limited data is available regarding insulin resistance amongst the rural Indian population [14].
In this scenario, the study has been done to investigate the prevalence of the insulin resistance amongst the undernourished rural population of the state West Bengal involving children, adolescents and adult volunteers. The effect of nutritional supplementation in the parameters echoing insulin sensitivity which may help to modify our existing maternal, adolescent and child nutritional supplement programs on long term basis to curb the epidemic of diabetes has also been investigated.
Materials and Methods
A prospective interventional study was performed in rural population of Malmar village, in the West Midnapore district of Bengal, India, over a period of six months starting from March 2015. Both male and female volunteers, aged over five years were randomly selected following a field study and proper written consent was obtained after explaining the methodology to be followed in local language. The consent of parents/guardians was taken in case of participants under the age of 18 years.
As per inclusion criteria, three age groups; Group 1 (5-12 years), Group 2 (13-18 years) and Group 3 (19-40 years), each with 40 subjects were designated for study. Sample size was determined based on the funding available for the project. Diagnosed cases of diabetes with or without treatment and pregnant females were excluded from the study according to the exclusion criteria. Nutritional status of the participants was assessed consistent with the criteria assigned for each age group. Specific parameters were allotted were checked before the study, in the middle and after the intervention in three groups during the six months study. Malnutrition was assessed by analysing weight for height (wasting) and height for age (stunting) in group1 and Body Mass Index (BMI) in group2 and group3.
Collection of Demographical Data and Intervention
Volunteers were interviewed for procuring demographical particulars, like age, socio-economic status, dietary pattern, family history including parental history of diabetes, and mental status relating to anxiety/depression with the aid of structured questionnaire. Medical history was evaluated in designed interviews comprising the use of prescription drugs. Subjects were also asked to give an account of the average duration of their daily physical activity and information on smoking habits or alcohol intake. Less than one hour activity per week was defined as low physical activity [15].
After the completion of general clinical examination, anthropometric details were collected. Body weight calculation was done in light clothing to nearest 1lb using a calibrated scale and height to the nearest 0.5 cm with a vertical ruler. Measurement of the waist circumference was done at the minimum abdominal girth and that of hip was assessed at the maximum protuberance of the hips at the level of the pubis symphysis to the nearest 0.1 cm [15]. BMI was gauged from by dividing weight to the square of height. Obesity is described in terms of abdominal adiposity as waist circumference exceeding the 80th sex-specific centile (men: >109 cm; women: >100 cm) and BMI more than or equal to 30 kg/m2 and underweight is defined as BMI <18•5 kg/m2 [16]. Wasting in Group 1 was classified as per Water Loo classification system of malnutrition in children where mild, moderate, severe as 80%-90%, 70%–80%, <70% weight for height of the children (z-scores/standard deviation below median weight for height). Stunting in the same group was classified as mild, moderate, severe as 90%-95%, 85%–90%, <85% height for age of the children [17,18].
Intervention involved the daily distribution of one ripe banana and an egg to the volunteers for consumption with the help of well instructed local agents. One ripe banana and egg is given in mid-day meal program in many rural areas of the West Bengal to reduce undernourishment. It wasn’t implemented in villages like Malmar. So the same meal supplement program was done in Malmar according to the project. Change in parameters like weight for height (Wasting), height for age (Stunting) and serum insulin was assessed initially and every three months in Group 1 for a duration of six months. BMI, serum insulin, FBS, IRI and HOMA-IR was evaluated in Group 2 and Group 3 in the beginning of the study and after six months [19].
Fasting blood samples were collected as per systematized CARDIA procedures and processed at qualified laboratories [20]. Quantitative measurement of fasting serum insulin was gauged by using Enzyme-Linked Immunosorbent Assay kit (Sigma Aldrich Inc., Missouri, USA) and that of fasting glucose level was done with auto analyser (Randox Daytona) by glucose oxidase method. Homeostasis model assessment was calculated using the formulae:
“HOMA-IR=Fasting glucose (mmol/l)×fasting insulin (µU/ml)/22.5” and Insulin Resistance Index by “IRI= Fasting glucose (mg/dl)×fasting insulin (µU/ml)/450” [19].
Statistical Analysis
Statistical analysis of the data was done by using Stata Statistical Software 9.0 (Stata Corp, Tex., USA). Analyses were accounted for sampling load, standard deviations and 95% confidence intervals. Descriptive results are expressed as mean±SD and p-value <0.05 were considered to be statistically significant [21]. Partial Least Square (PLS) which is suitable for a smaller sample size due to its partial nature was used for model testing as it doesn’t make restrictive assumptions about the data distribution.
Results
Age Group 1
Change in the nutritional status of the volunteers depicted by improvement in weight for height, height for age, serum insulin and FBS is given in [Table/Fig-1,2,3 and 4].
Change in nutritional status of the volunteers in Group 1 over six months of supplementation study.
| Parameter | Zero Month | Third Month | Sixth Month |
|---|
| Normal weight for height | 19.69± 2.3% | 53.03±3.1% | 66.67±5.4% |
| Mild wasting | 21.22±1.2%, | 19.69%±3.1% | 27.27±1.9% |
| Moderate wasting | 30.30±2.2% | 24.24%±1.5% | 6.06±1.1% |
| Severe wasting | 28.79± 2.8% | 3.03%±.9% | nil |
| Normal height for age | 23.21±1.1% | 28.79% ±2.3% | 33.33±2.4% |
| Mild stunting | 25.27±1.7% | 24.24%±2.9% | 25.76±1.3% |
| Moderate stunting | 28.3±2.5% | 28.79%±2.2% | 27.27±2.9% |
| Severe stunting | 23.22±2.1% | 16.67%±1.7% | 13.64±1.1% |
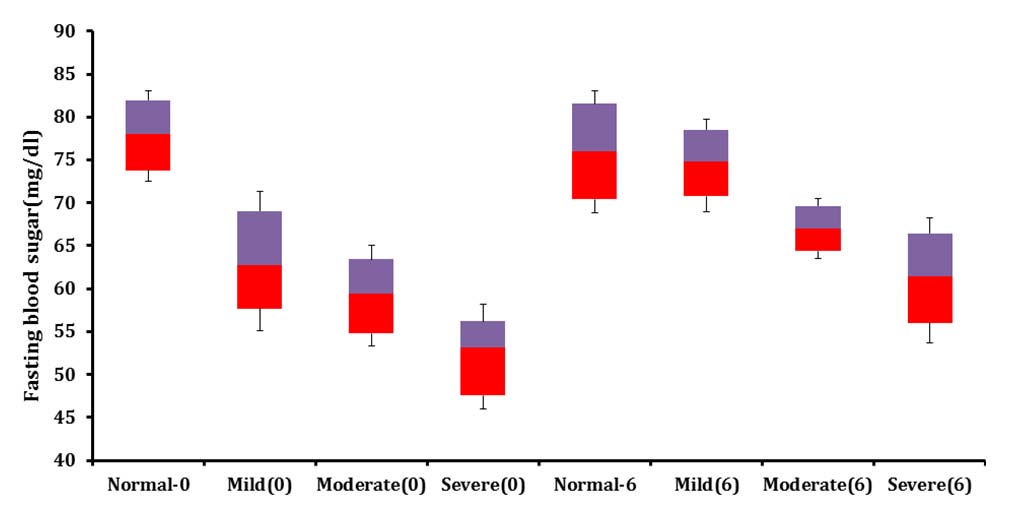
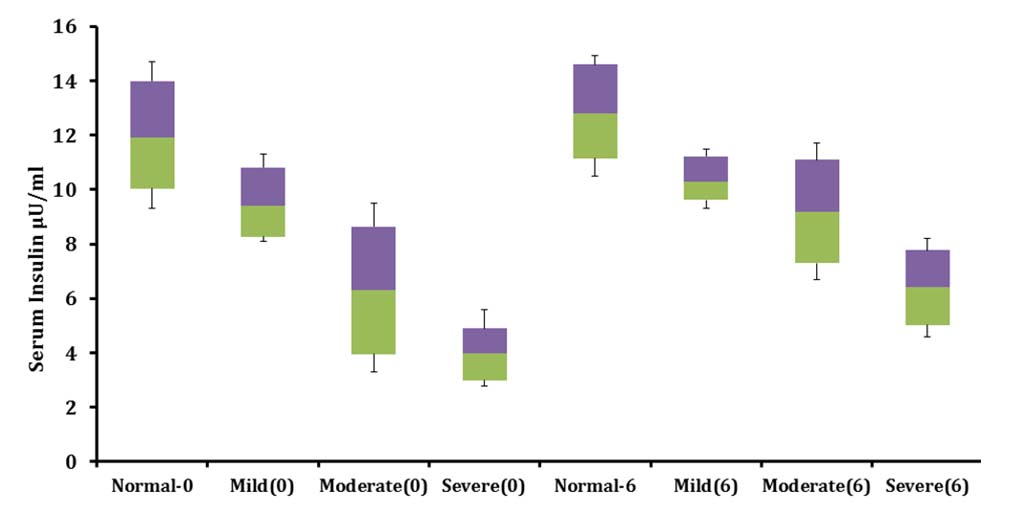
Shows change in serum insulin and fasting blood sugar over six months of supplementation study.
| Parameter | Serum insulin(µU/ ml) Zero month | Serum insulin (µU/ ml) Sixth month | Fasting blood sugar (mg/dl) Zero month | Fasting blood sugar (mg/dl) Sixth month |
|---|
| Normal weight for height | 12±2.7 | 12.8±2.11 | 78.08±5.6 | 76±7.1 |
| Mild wasting | 9.4±1.9 | 10.4±1.1 | 63.25±8.1 | 74.8±4.8 |
| Moderate wasting | 6.3±3.2 | 9.2± 2.6 | 59.4±5.7 | 67.0±3.5 |
| Severe wasting | 4.0±1.6 | 6.4±1.9 | 52.1±6.1 | 61±7.3 |
Shows improvement in fasting blood sugar in three sub clusters classified as per the degree of malnutrition in age Group 1 over six months of feeding program.
Shows Change in fasting serum insulin levels in three sub clusters classified as per the degree of malnutrition in age Group 1 over six months of feeding program.
Age Group 2
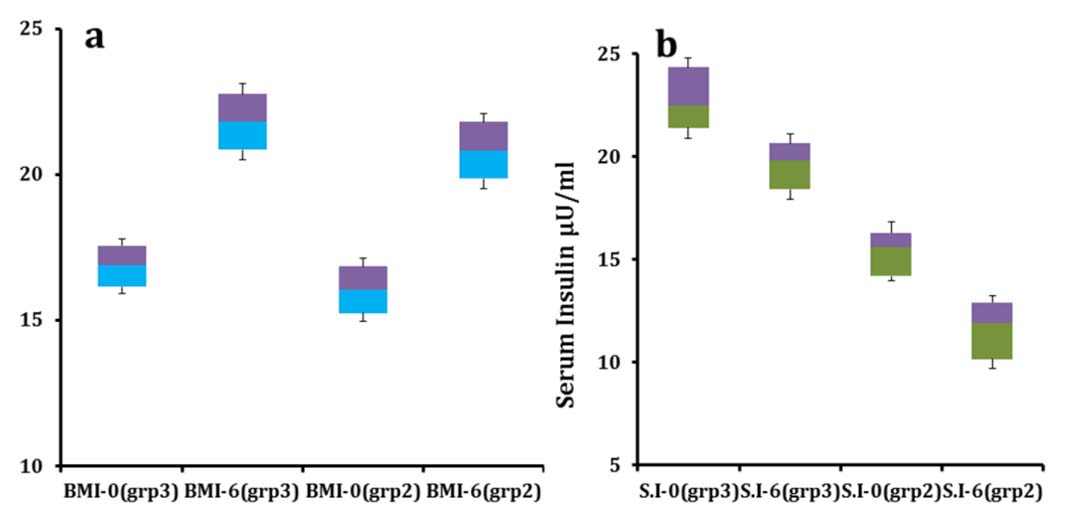
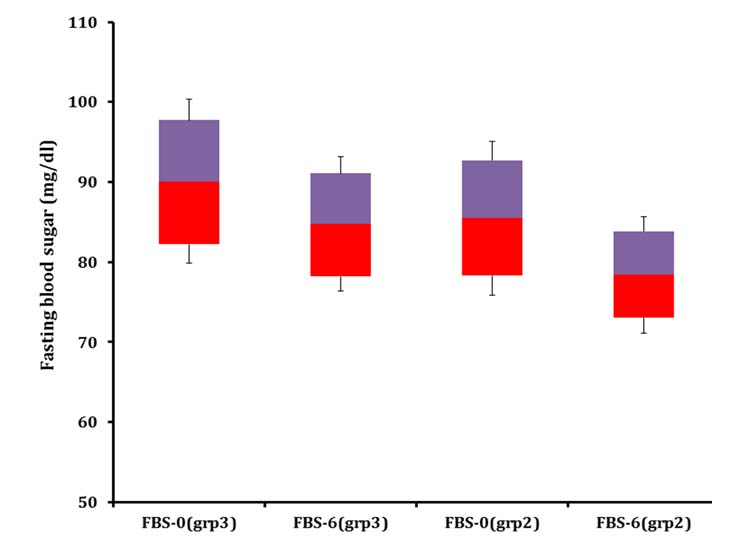
Average BMI of the adolescent volunteers augmented from 16.04±1.1 to 20.8±1.3 with a percentage increment of 31.2% [Table/Fig-5a] and mean serum insulin level reduced from 15.1±2.22 µU/ml to11.4±2.42 µU/ml (p<0.05) after feeding [Table/Fig-5b]. Average FBS enhanced from 85.5±9.6 mg/dl to 78.4±7.3 mg/dl [Table/Fig-6]. HOMA-IR and IRI values of the adolescents improved from the initial figures (3.19, 2.87) to (2.204, 1.99) respectively over six months.
(a & b). Shows change in BMI and serum insulin respectively in age Group 2 and 3 over six months of feeding program.
grp2= Group 2, grp3= Group 3
Shows change in fasting blood sugar in age Group 2 and 3 over six months of feeding program.
grp2= Group 2, grp3= Group 3
Age Group 3
Average BMI of the adult volunteers improved from 16.9±0.9 to 21.8±1.3 with a fractional growth of 29.5% and that of serum insulin decreased from 22.6±2.7 µU/ml to 19.5±2.1 µU/ml (p<0.05) after the feeding. Mean FBS improved from 90.1±10.2 mg/dl to 84.8±8.4 mg/dl by the end of the supplementation study. HOMA-IR and IRI values of the adult volunteers changed from (5.0, 4.52) to (4.08, 3.67) correspondingly by the end of the study.
Discussion
This is the first population-based study on the rural population of the West Bengal to study the relation between malnutrition and insulin resistance. The percentage of children with undernourishment was reduced at the end of the study, indicating the efficiency of the supplements employed in improving the nutritional status. This is supported by the elevation in the FBS and serum insulin levels in the group 1 alongside the nutritional enhancement, in efforts to restore body metabolism. Parameters like serum insulin, FBS, HOMA-IR and IRI was found to be decreased and BMI was found to be decreased in adolescent and adult volunteers by the end of the study, evocative of renovation in insulin sensitivity.
In the developing sectors of the world, stunting and wasting is allied with maternal malnutrition, insufficient quality or quantity of complementary nourishments during infancy, reduced absorption of nutrients instigated by parasitic infestation and malabsorption syndromes [22]. The fasting insulin level in malnourished children was perceived to be low when compared to that of healthy counterparts which may be an adaptive response, to reinstate blood glucose levels, ensuring continuous fuel supply to the vital organs. Insulin-secretory response of the pancreatic beta cells is also found to be low, consequently reduced insulin glucose ratio suggesting poor cell function in malnourished subjects. It is unlikely that structural damage is a major causative factor since the abnormalities disappear following the recovery [23,24]. Protein malnutrition brings about β cell mitochondrial dysfunction, piloting to an increased AMP PATH mediated insulin secretion owing to the enhanced glutamate dehydrogenase content and activity [25]. Childhood malnutrition is suggested to be linked with alterations in glucose, insulin, lipids metabolism that contributes to non-communicable diseases and associated low serum insulin level is often associated with hypoalbuminaemia, potassium deficiency and concurrent infection leading to stunted growth [26]. Association between the impaired glucose tolerance and chronic undernourishment is backed by the animal studies where protein and energy restriction resulted in glucose intolerance. Comparable findings is reported in the children suffering from Protein Energy Malnutrition (PEM) and in adults with moderate to severe undernourishment [27].
Certain nutrient deficiencies seen in malnutrition have alliance with glucose intolerance, amongst them pyridoxine, chromium, vanadium, thiamine, vitamin D and biotin have received ample attention in the development of diabetes mellitus [28-31]. Malnourished populace in several nations are found to be deficient in chromium and energy intake of less than 2000 kcal is most likely associated with an undesirably low chromium intake [29]. Studies have exhibited that chromium bound to the oligopeptide chromodulin improve tyrosine kinase activity of the insulin receptor, simultaneously impeding phospho-tyrosine phosphatase activity [31]. Association between low serum intracellular magnesium concentrations seen in undernourishment and decreased insulin secretion coupled with insulin resistance leading to Type 2 diabetes mellitus is evidenced in big epidemiologic studies. Urinary excretion of magnesium is often proposed to be associated with insulin resistance, further decreasing the serum levels. The role of magnesium deficiency in the occurrence of glucose intolerance during infancy and childhood is poorly understood [30]. Thiamine is a vital micronutrient interceding several key enzymes of glycolysis, the citric acid and the pentose phosphate shunt pathways. Moderate thiamine deficiency is reported in 17%–79% of diabetic patients and enduring studies is essential to explore its potential use to amend the course of diabetes mellitus [31].
Studies have described lower levels of biotin in patients with type 2 diabetes mellitus in comparison to healthy controls. Mechanism of action is suggested to be due to biotin induced hexokinase gene expression that improves hepatic glucose uptake. This is defended by animal studies suggesting the growth of insulin resistance in biotin lacking underfed rats which is resolved by biotin replacement [31]. Vitamin D standardizes nuclear peroxisome proliferative activated receptor which has an imperious role in insulin sensitivity and moderates the levels of proinflammatory cytokines involved in insulin resistance like as interleukins, via the β cell vitamin D receptors, 1α hydroxylase on islet cells, vitamin D response element in insulin gene and skeletal muscle [32].
Gonza´lez-Barranco J et al., established that irrespective of birth weight, malnutrition has antagonistic effects on insulin metabolism and glucose tolerance in young males [27]. Comparatively high FBP in underweight in comparison to that of individuals with normal BMI despite the corresponding insulin secretion is reduced by the end of the supplementation project reflecting insulin resistance at receptor level [25]. This is also supported by recuperated insulin sensitivity echoed by improved IRI and HOMA-IR values. Exposure to poor nutrition like that of famine in early life is associated with the development of the Type 2 diabetes mellitus in later life [33,34].
Limitation
Study is done in a small populace, definite conclusion prerequisites big sample size from different regions and ethnic groups. Heterogeneity in lifestyle, physical activities and food intake was not taken into account, since the study was done in an open population.
Conclusion
Nutritional supplementation is suggested to have a vital role in preventing and restoring protein energy malnutrition leading to insulin resistance and hence Type 2 diabetes mellitus. Low values of parameters like serum insulin, FBP, HOMA-IR and IRI reflects improvement in insulin sensitivity status with supplementary nutrition. The present study make a foundation for more elaborate studies on malnourishment linked insulin resistance and highlights the need for maternal and child nutritional supplement programmes in the primary care centres and adolescent programmes on long term basis to curb the epidemic of insulin resistance leading to glucose intolerance in rural societies.