In India, the number of new gastric cancer patients is approximately 34000 each year with male to female ratio of 2:1 [1]. Gastric carcinoma is the second most common cause of cancer-related deaths in both men and women in India [2].
Human epidermal growth factor receptor 2 (HER2) is a proto-oncogene present on chromosome 17. The HER2 has transm-embrane tyrosine kinase activity that promotes cell proliferation and suppresses apoptosis thereby leads to tumorigenesis. HER2 is involved in the pathogenesis and poor outcomes of a subset of breast and advanced gastric cancers [3]. After the recent approval of Trastuzumab (anti-HER2 antibody) for the treatment of HER2 overexpressed GA, importance of HER2 testing is increasingly recognized. There are fair numbers of studies available worldwide on HER2 status in gastric cancer patients [3]. However, data regarding HER2 expression in the Indian gastric cancer patients is available in only a limited number of studies [4–6].
Genetic alterations are known to play pivotal role in pathogenesis of cancers including carcinoma of the stomach. Among these, the most frequent change is observed in the p53 gene [7]. Normally, p53 gene encodes wild-type p53 protein, which has a very short half-life, and controls the cell cycle (G1 phase arrest) and apoptosis of cells with damaged DNA [8]. Point mutation, deletion, or rearrangement of p53 gene causes formation of mutant p53 protein [9]. Mutant form of p53 protein is very stable and can accumulate in the nuclei of tumour cells [10]. IHC staining with specific antibody can be used to detect mutant p53 protein. However, only a few studies are available for p53 expression in the Indian patients of gastric carcinoma [11].
In this study, we evaluated IHC expression of HER2 and p53 in GA of North Indian patients. We also correlated expression of HER2 and p53 with each other and with demographic profile and histological types of GA.
Materials and Methods
This was an observational and analytical study carried out at Institute of Medical Sciences, Banaras Hindu University, Varanasi, India. The study was approved by Institutional Ethics Committee.
H&E: We examined 50 endoscopic-guided gastric biopsy cases diagnosed as adenocarcinoma between September 2013 and August 2014. Age and sex of the cases were noted. Haematoxylin and Eosin (H&E) stained slides of the cases were retrieved, reviewed, and they were classified according to Lauren classification into diffuse, intestinal, or indeterminate types.
HER2: Immunohistochemical study for HER2 was performed on formalin-fixed paraffin embedded (FFPE) tissue blocks. The HER2 antibody was a primary monoclonal rabbit antibody (clone SP3, Thermo Scientific, USA, ready to use). Secondary detection horse-radish peroxidase kit was procured from Biogenex, USA. Positive control for HER2 was a known HER2 overexpressed case of infiltrating ductal carcinoma of the breast. The scoring method for HER2 is given in [Table/Fig-1] [12].
Scoring method for HER2 immunohistochemistry in the gastric biopsy samples.
| HER2 Score | Gastric biopsy specimen staining pattern | HER2-interpretation |
|---|
| 0 | No reactivity or no membranous reactivity in any tumour cell | Negative |
| 1 | Tumour cell cluster with a faint/barely perceptible membranous reactivity irrespective of percentage of tumour cells stained | Negative |
| 2 | Tumour cell cluster with a weak to moderate complete, basolateral, or lateral membranous reactivity irrespective of percentage of tumour cells stained | Equivocal |
| 3 | Tumour cell cluster with a strong complete, basolateral, or lateral membranous reactivity irrespective of percentage of tumour cells stained | Overexpression |
p53: Immunohistochemical studies were performed on FFPE blocks using anti-p53 protein primary monoclonal mouse antibody (clone DO7, Biogenex Inc, USA, ready to use). Secondary horseradish peroxidase kit was procured from Biogenex, USA. Positive control for p53 was a known positive case of infiltrating ductal carcinoma of the breast.
The scoring of p53 was made using following criteria [13]: negative (0-9% tumour cell nuclei positive) and positive (10% or more tumour cell nuclei positive). Positive results of p53 were scored as either weak or strong.
Statistical Analysis
Descriptive analysis was done for this study.
Results
Demography : Out of total 50 cases of GA, mean age of the patients was 56.8±14.8 years. Age range of the patients was 25-80 years. Male: female ratio was 2:1.
Histopathology: Various histological types of GA were: intestinal (34 cases/68%), diffuse (14 cases/28%) and indeterminate type (2 cases/4%).
HER2: HER2 expression score of 0, 1+, 2+, and 3+ were present in 28 cases (56%), 12 cases (24%), 5 cases (10%) and 5 cases (10%), respectively [Table/Fig-2a-c]. Four cases of intestinal type and a single case of indeterminate type histology showed HER2 overexpression (score 3+). HER2 expression was negative in all the cases of diffuse type histology.
Diaminobenzidine chromogen stained immunohistochemical microphotographs of intestinal type of gastric adenocarcinoma showing: (a) Faint barely perceptible staining (score 1) in the glands (HER2, 40X); (b) Incomplete membranous staining (score 2) in the glands (HER2, 40X); (c) Complete membranous staining (score 3) in the glands (HER2, 40X); (d) Strong nuclear expression in more than 90% nuclei (p53, 40X).
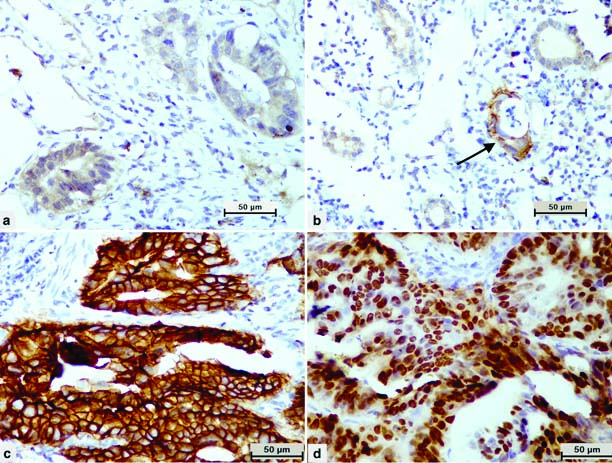
p53: Positive nuclear expression of p53 was seen in 36 cases (72%) [Table/Fig-2d]. Strong and weak intensity of p53 expression was present in 24 cases (48%) and 12 cases (24%), respectively. p53 expression was seen in 27 cases of intestinal type (mean p53 expression 43.3%) and seven cases of diffuse type (mean p53 expression 27.8%) histology. In two cases of indeterminate type histology, p53 expression was 60% and 70%.
Correlation of HER2 and p53 expression: Statistically significant correlation was not found between immunohistochemical expression of HER2 and p53 in GA of this study. However, HER2 equivocal (score 2+) and overexpressed (score 3+) cases together showed a higher percentage of p53 positivity than HER2 negative (score 0 and 1) cases [Table/Fig-3].
Correlation of HER2 expression with p53 expression in gastric biopsy samples (n=50).
| Variables | p53 expression |
|---|
| Negative | Positive |
|---|
| HER2 expression | Negative | 13 (32.5%) | 27 (67.5%) |
| Equivocal/overexpression | 1 (10.0%) | 9 (90%) |
Discussion
Testing for HER2 has now become a routine practice in guiding treatment and prognosis in breast cancer. The recommended methods for assessing the HER2 status are gene amplification using Fluorescence In-Situ Hybridization (FISH), and protein overexpression using Immunohistochemistry (IHC), or both. Out of these two methods, IHC is the primary method of choice for assessment of HER2 status in breast and gastric cancers. This is due to cost-effective set up of IHC and relative ease of performing the test. In comparison to IHC, the setup and cost for FISH testing is expensive and requires expertise in both performance and interpretation of the test. Moreover, there are guidelines that HER2 score of 2 (equivocal result) should be further tested by FISH [3].
There is wide geographical variation in the incidence rate of gastric cancer within India; the highest occurrence in North-eastern and South Indian states [1]. In India, HER2 testing in gastric cancer has been done in only few studies, in which overexpression of HER2 was seen in 44.2%, 9.6%, 21.4% and 26.6% cases [4–6,14]. In comparison to these studies, we have observed overexpression of HER2 in 10% cases. All of these results of HER2 overexpression in Indian patients of gastric cancer needs further validation by FISH.
Mutations/polymorphisms in p53 gene by PCR have been depicted in Indian patients with gastric cancer [10,15–20]. Mutations involving exons 5,6,7, and 8 region of p53 gene were observed in a handful of Indian studies in 18.4%, 20.9%, and 46% cases [10,16,17]. The other two Indian studies found single nucleotide polymorphism of p53 gene involving exon 4 [18,20]. Polymorphism of p53 gene in the region of intron 3 Ins16bp was studied by Malik MA et al., [15]. Immunohistochemistry for p53 protein has been performed in only two Indian studies with an identical positive p53 result of 35.9% in both of them [10,19]. We observed positive expression of p53 in 72% cases of gastric cancer in our study. Higher p53 expression of our study can be ascribed to lower cut-off of 10%, which we have taken from the study of Rosales-Pérez S et al., [13]. However, considering cut-off at 25% similar to other two Indian studies, we will get positive p53 expression in 40% cases. As seen in a previous study, we have also observed a higher expression of p53 protein in intestinal type than diffuse type of GA [10].
On comparing the result of p53 between PCR and immunohi-stochemistry, it was noticed that higher number of p53 expression is detected by immunohistochemistry (18.4% vs. 35.9%) [10]. This indicates that there are mutations involving other regions of p53 gene. Nevertheless, our study indicates that p53 mutation is present in sizable numbers of Indian gastric cancer patients. This observation requires further investiagations by PCR involving other exonic sequences of p53 gene.
Mutation in the exon 5-8 of p53 gene by PCR of gastric tissue was observed in 4.6% of general population, however, gastric cancer patient harbored this mutation in 21%, while K-ras gene codon 12 mutation was not seen in Indian patients with gastric cancer [16]. In a study from Mizoram, tobacco-smoking has been found as an important risk factor for high incidence of gastric cancer [18]. Analysis of p53 72 single nucleotide polymorphism in 16 cases of gastric cancer revealed that Pro/Pro, Arg/Arg, and Arg/Pro variants were seen in 5, 4 and 7 cases, respectively [20]. In a study, on 103 Indian patients (collected from Kolkata, Shrinagar and New Delhi) of gastric cancer, male: female ratio was found to be 3.1:1. Expression of p53 protein in gastric cancer by immunohistochemistry and mutation of p53 gene (exon 5,6,7,8) by PCR was found in 35.2% and 18.4%, respectively [10]. A higher positivity for p53 mutation was observed in intestinal type (22.4%) as compared to diffuse type of GA (15.66%) [10]. While 21% carcinomas were immunopositive for p53 by IHC, 4% of mutant p53 by PCR did not stain by IHC [10]. As observed in various cancers, role of p53 lies in causation of GA. It has been observed that chronic infection with Helicobacter pylori augments p53 mutations with resultant excessive proliferation and impaired apoptosis, which increases the risk of GA. Thus, detection of p53 may be used as a potential marker for identification of Helicobacter pylori associated gastric cancers [21].
Limitation
Our study is limited by small sample size and lack of correlation between IHC markers (HER2 and p53) and cancer stage as well as follow-up of patients in terms of response to treatment and survival.
Conclusion
In comparison to HER2 negative cases, there appears some role of p53 in HER2 equivocal and overexpressed gastric cancer. Overall, p53 expression was seen in substantial number of gastric cancer in our study. Our findings highlight the need for searching additional mutation sites in p53 gene in gastic cancer. Immunohistochemical and molecular study on larger number of samples with clinical correlation and follow-up data from different parts of the country will further our knowledge regarding diagnostic/prognostic roles of p53 and HER2 in Indian patients of gastric cancer.