Tumoural angioneogenesis and its quantification are important in predicting the tumour grade and its management with respect to the treatment available and also in evaluating the prognosis and response to treatment. It also plays major role in the growth and spread of tumours [1]. Hence, there is a need for non-invasive in vivo methods to assess tumour angioneogenesis and tumour grade at the time of presentation and for monitoring the response during treatment and follow-up [2]. In this regard PCT can be easily added into routine CT studies to obtain information on lesion physiology along with its morphology. It allows absolute quantification of the physiological parameters which include the blood flow, blood volume, mean transit time, time to peak etc., which stand as physiological representatives of microscopic changes occurring in the tumour [3,4].
The advantages of using CT method of quantification include simplicity, wide availability and reproducibility [5]. It has also been validated against H215O-PET [6]. As compared to the MR perfusion, quantification in CT perfusion is simpler as the attenuation values and the contrast concentration bear a more linear relationship [5]. MR perfusion is often limited by the availability of MRI machine, and is more expensive with problems of artifacts in postoperative patients.
We aimed to prospectively evaluate the efficacy of perfusion CT in differentiating the intracranial space occupying lesions.
Materials and Methods
This was a prospective study conducted in Kasturba Hospital, Manipal, Karnataka, India, between September 2009 and September 2011. Since it was done for a short period of two years, all the consenting patients having intracranial space occupying lesions (both intra-axial and extra-axial) were included in the study. This amounted to a total of 136 patients. Institutional Ethical Committee approval was obtained.
Out of the 136 patients, 68 patients having lesions of interest (17 high-grade gliomas, 10 low-grade gliomas, 7 lymphomas, 27 metastases and 7 abscess) were considered for the present study.
Written consent was obtained from the patients in English or local language. Inclusion criteria for the study were: (a) patients who had not undergone biopsy or treatment before the study; (b) patients whose final diagnosis was confirmed by histopathology in case of a neoplasm, known primary in case of metastases and response to appropriate antibiotic treatment in case of an infective lesion. Patients with impaired Renal Function Tests (RFTs), past history of significant allergy to iodinated contrast media and cases in whom intracranial hematomas formed the space occupying lesion were excluded from the study. Departmental research funds were utilized for the study without any extra cost to the patients.
CT Perfusion Imaging
Perfusion imaging was performed using 64 slice Philips CT machine. The parameters were 80 kV and 100 mAs, 5 mm slice thickness, gantry rotation time of one second.
Initially axial CT scan of brain was obtained without contrast. The lesion was localized and a 4 cm scan area was selected such that the maximum epicenter of the lesion was included within the area. Almost 50 ml of non ionic iodinated contrast medium (300 mg iodine/ml) was injected at the rate of 4 ml/sec through an 18 gauge i.v. canula. Image acquisition was done in cine (continuous) mode starting at four seconds of beginning of contrast injection. A total of 40 cycles were run keeping an intercycle interval of 1.0 second. Each cycle yielded eight images with each image having 5 mm thickness.
Post Processing and Image Analysis
Each study yielded a total of 320 images which were processed in Philips Extended Brilliant Workstation using CT brain perfusion software. Radiological analyses and interpretation were performed by two experienced radiologists who were blinded to the patient’s clinical details and identity.
The anterior cerebral or middle cerebral artery was auto-selected by the software as input artery and superior sagittal sinus was auto-selected as venous input. Functional colour maps were generated (colour ranging from blue to red) for qualitative assessment of perfusion parameters.
Region of Interest (ROI) was placed over the tumour region in the enhancing part of tumour using parametric colour maps as a guide, without including areas of necrosis, cystic areas and areas with major cortical vessels and the so obtained perfusion parameters were labeled as absolute values. ROI was also placed in the normal brain parenchyma over the contra-lateral hemisphere and obtained perfusion parameters were labeled as normal values.
For each patient, four parameters were calculated viz., CBV-ml/100g, CBF – ml/100g/min, MTT - seconds and TTP- seconds. Absolute values were divided by the normal values to obtain the normalized ratios of CBV (nCBV), CBF (nCBF), MTT (nMTT) and TTP (nTTP).
Statistical Analysis
Normalized parameters obtained were correlated with the final diagnosis as confirmed by the set gold standard. Findings were tabulated and statistical analysis (student independent t-test and ROC analysis) was performed using SPSS 17.0 software. The means of the perfusion parameters of various lesions was obtained and compared using student independent t-test for statistical significance.
The perfusion parameters that showed statistically significant difference in differentiating two types of lesion were identified and the Receiver Operating Characteristic (ROC) curves for these parameters were obtained. The generated ROC curves were used to obtain the cut off points that increase the sensitivity and specificity in identifying a lesion.
Results
Demographic and clinical details are shown in the [Table/Fig-1]. Mean age of presentation was 56.3±12.4 years. Comparison of the mean, minimum value, maximum value, and standard deviation of the perfusion parameters between different histopathological subgroups is shown in the [Table/Fig-2].
Demographic and clinical details of the cases.
| Parameters | Number | Percentage |
|---|
| Male | 40 | 58.8 |
| Female | 28 | 41.2 |
| High grade gliomas | 17 | 25.0 |
| Low grade gliomas | 10 | 14.7 |
| Lymphomas | 07 | 10.3 |
| Metastasis | 27 | 39.7 |
| Abscess | 07 | 10.3 |
Statistical values of various perfusion parameters in different histopathological subgroups.
| Diagnosis | nCBV* | nCBF† | nMTT‡ | nTTP§ |
|---|
| High Grade Glioma | Mean | 3.8482 | 2.4706 | 1.5088 | 1.0904 |
| N | 17 | 17 | 17 | 17 |
| Std. Deviation | 0.85823 | 0.52762 | 0.48806 | 0.10884 |
| Minimum value | 2.52 | 1.12 | 0.93 | 0.96 |
| Maximum value | 5.07 | 3.39 | 2.60 | 1.35 |
| Low Grade Glioma | Mean | 2.0400 | 1.8710 | 1.7240 | 1.0630 |
| N | 10 | 10 | 10 | 10 |
| Std. Deviation | 0.61507 | 0.43033 | 0.54716 | 0.07166 |
| Minimum value | 1.17 | 1.23 | 0.96 | 0.99 |
| Maximum value | 3.03 | 2.55 | 2.11 | 1.19 |
| Primary CNS Lymphoma | Mean | 2.7143 | 2.8971 | 2.3429 | 1.3957 |
| N | 7 | 7 | 7 | 7 |
| Std. Deviation | 0.52009 | 1.37512 | 0.34937 | 0.64727 |
| Minimum value | 1.94 | 1.64 | 1.86 | 1.09 |
| Maximum value | 3.38 | 5.22 | 2.94 | 2.86 |
| Metastasis | Mean | 7.3373 | 4.2004 | 1.9915 | 1.1063 |
| N | 27 | 27 | 27 | 27 |
| Std. Deviation | 5.80309 | 3.65065 | 0.82312 | 0.24726 |
| Minimum value | 0.04 | 0.02 | 0.79 | 0.44 |
| Maximum value | 20.11 | 15.89 | 4.15 | 1.76 |
| Abscess | Mean | 3.3571 | 1.8386 | 2.0514 | 1.1243 |
| N | 7 | 7 | 7 | 7 |
| Std. Deviation | 1.22462 | 0.90733 | 0.98455 | 0.14129 |
| Minimum value | 1.40 | 0.92 | 1.02 | 0.94 |
| Maximum value | 4.88 | 3.35 | 3.82 | 1.30 |
- normalized Cerebral Blood Volume,
- normalized Cerebral Blood Flow,
- normalized Mean Transit Time,
- normalized Time To Peak.
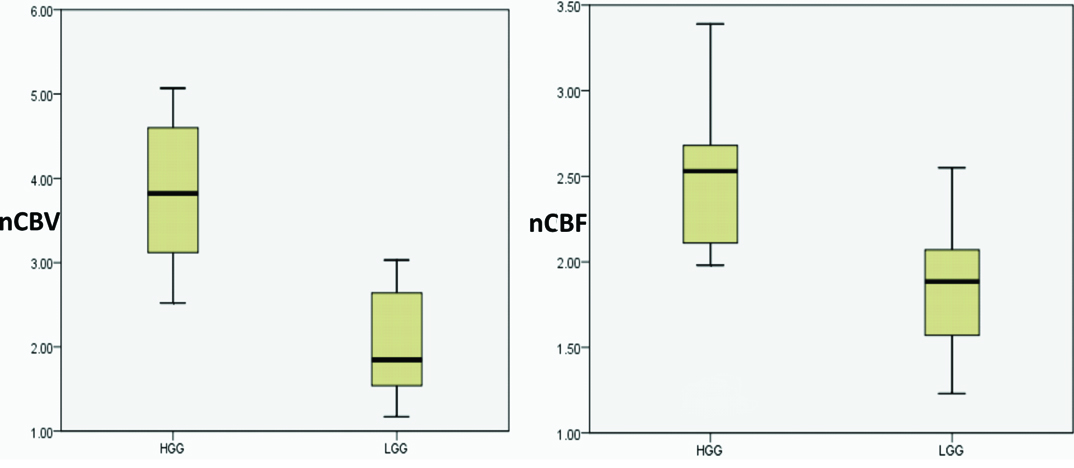
Statistical comparison of high grade and low grade gliomas: Perfusion parameters that showed statistically significant difference (<0.05) indifferentiating high grade from low grade gliomas were mean nCBV and nCBF. Mean nMTT and nTTP showed no statistically significant difference in differentiating these lesions [Table/Fig-3]. [Table/Fig-4] represents the boxplot showing the range of nCBV and nCBF among the low grade and high grade gliomas and [Table/Fig-5] shows their ROC curves.
The p-values for the perfusion parameters in differentiating high grade gliomas from low grade gliomas, primary CNS lymphomas, metastases and abscess.
| Variables | nCBV * | nCBF† | nMTT‡ | nTTP§ |
|---|
| HGG|| vs. LGG** | 0.000015 | 0.004 | 0.797 | 0.439 |
| HGG vs. PCNSL†† | 0.006 | 0.274 | 0.002 | 0.065 |
| HGG vs. Metastases | 0.049 | 0.060 | 0.042 | 0.771 |
| HGG vs. Abscess | 0.312 | 0.284 | 0.549 | 0.452 |
- normalized Cerebral Blood Volume,
- normalized Cerebral Blood Flow,
- normalized Mean Transit Time,
- normalized Time To Peak,
- High Grade Glioma,
- Low Grade Glioma,
- Primary CNS Lymphomas
Boxplot showing the range of nCBV and nCBF among low- and high-grade tumours (HGG: High Grade Glioma, LGG: Low Grade Glioma).
ROC curves showing the area under the curve for nCBV (A) and nCBF (B) in differentiating high grade gliomas and low grade gliomas.
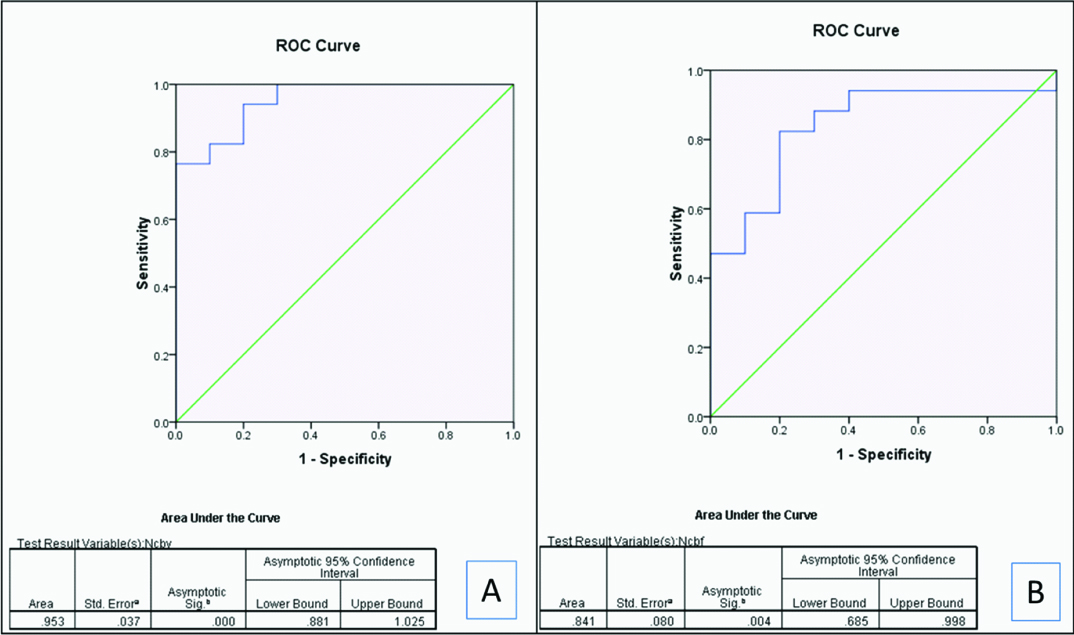
A cut off of > 3.07 for nCBV yielded 76.5% sensitivity and 100% specificity and > 2.08 for nCBF yielded 82.4 % sensitivity and 80% specificity in identifying high-grade gliomas [Table/Fig-6].
Sensitivity and specificity of mean nCBV and nCBF to identify high grade gliomas.
| Cut off | Sensitivity | Specificity |
|---|
| nCBV* >3.07 | 76.5% | 100% |
| nCBF†>2.08 | 82.4% | 80% |
- normalized Cerebral Blood Volume,
- normalized Cerebral Blood Flow
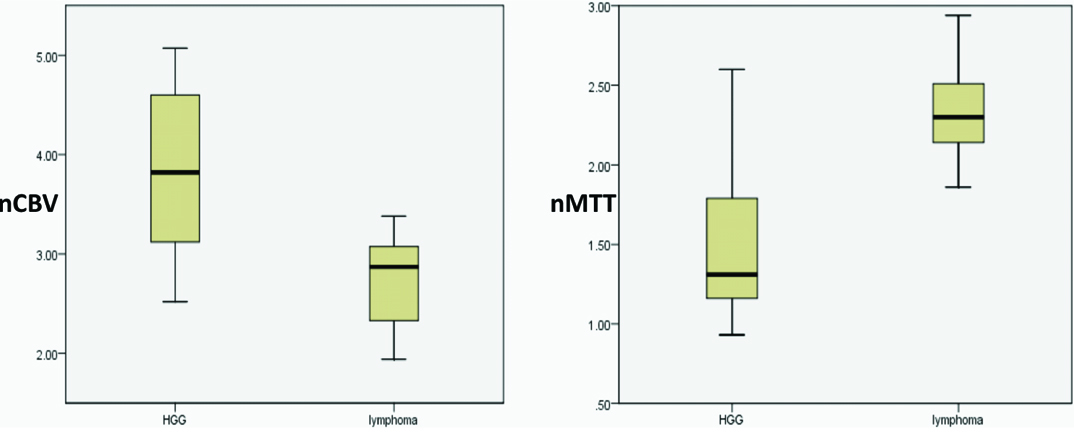
Statistical comparison of high grade gliomas and primary CNS lymphomas (PCNSLs): Perfusion parameters that showed statistically significant difference (<0.05) in differentiating the two lesions were mean nCBV and nMTT. Mean nCBF and nTTP showed no statistically significant difference in differentiating these lesions [Table/Fig-3]. [Table/Fig-7] represents the boxplot showing the range of nCBV and nMTT among the high grade gliomas and lymphomas and [Table/Fig-8] shows their ROC curves.
Boxplot showing the range of nCBV and nMTT among high-grade gliomas and lymphomas (HGG: high grade glioma).
ROC curves showing the area under the curve for nCBV (A) and nMTT (B) in differentiating high grade gliomas and lymphomas.
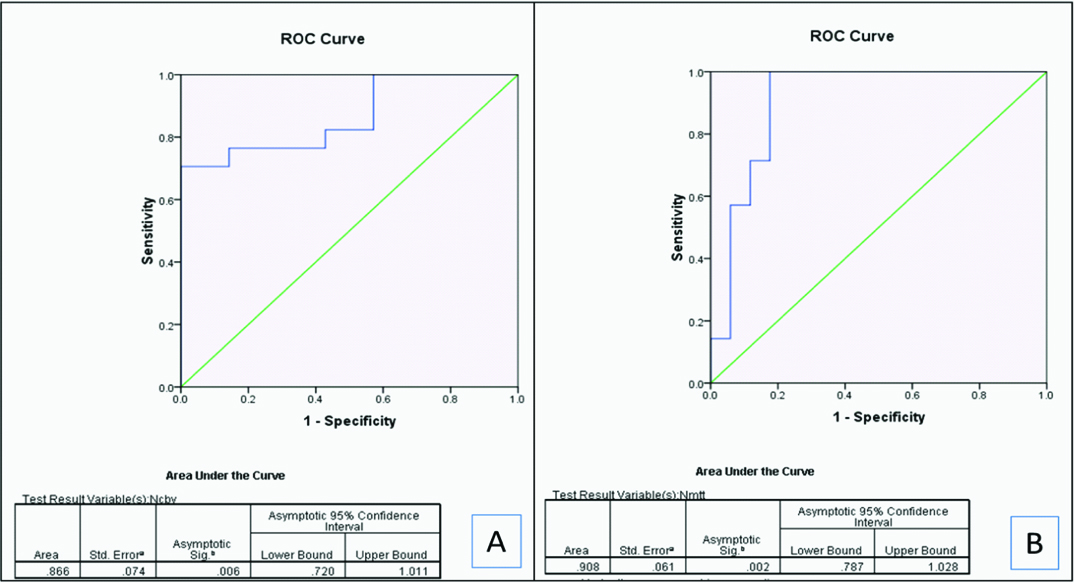
A cut off of <3.40 for nCBV yielded 100% sensitivity and 70.6% specificity and >1.83 for nMTT yielded 100% sensitivity and 82.4 % specificity in identifying lymphomas [Table/Fig-9].
Sensitivity and specificity of mean nCBV and nMTT to identify Lymphomas.
| Cut off | Sensitivity | Specificity |
|---|
| nCBV* <3.40 | 100% | 70.6% |
| nMTT†>1.83 | 100 % | 82.4% |
- normalized Cerebral Blood Volume,
- normalized Cerebral Blood Flow
Statistical comparison of high grade gliomas and metastases: Breast carcinoma (n=9), bronchogenic carcinoma [n=6], renal cell carcinoma (n=5), follicular carcinoma of thyroid (n=3), colorectal carcinoma (n=2), malignant melanoma (n=1) and neuroendocrine tumour of pancreas (n=1) formed the primaries for a total of 27 cases with metastases.
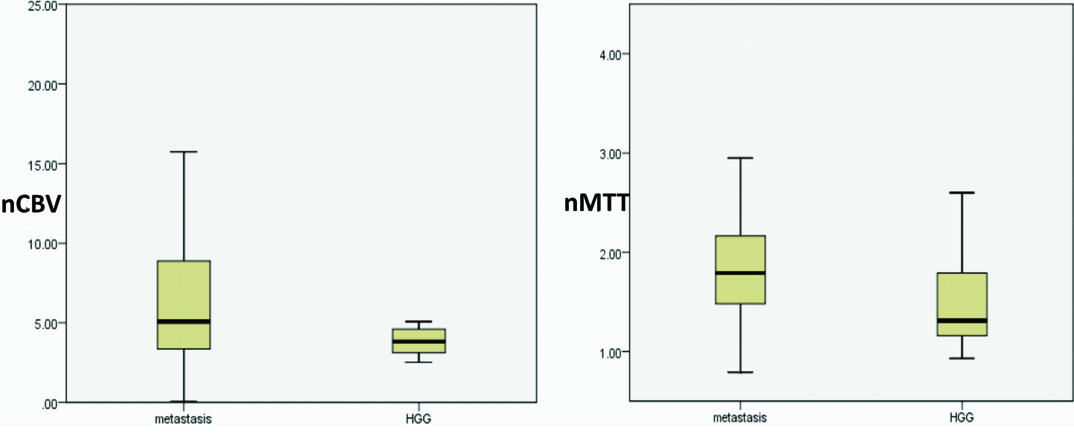
Perfusion parameters that showed statistically significant difference (< 0.05) in differentiating high grade gliomas from metastatic lesions were mean nCBV and nMTT. Mean nCBF and nTTP showed no statistically significant difference in differentiating these lesions [Table/Fig-3]. [Table/Fig-10] represents the boxplot showing the range of nCBV and nMTT among the high grade gliomas and metastases and [Table/Fig-11] shows their ROC curves.
Boxplot showing the range of nCBV and nMTT among metastasis and high-grade gliomas (HGG: high grade glioma).
ROC curves showing the area under the curve for nCBV(A) and nMTT (B) in differentiating high grade gliomas and metastases.
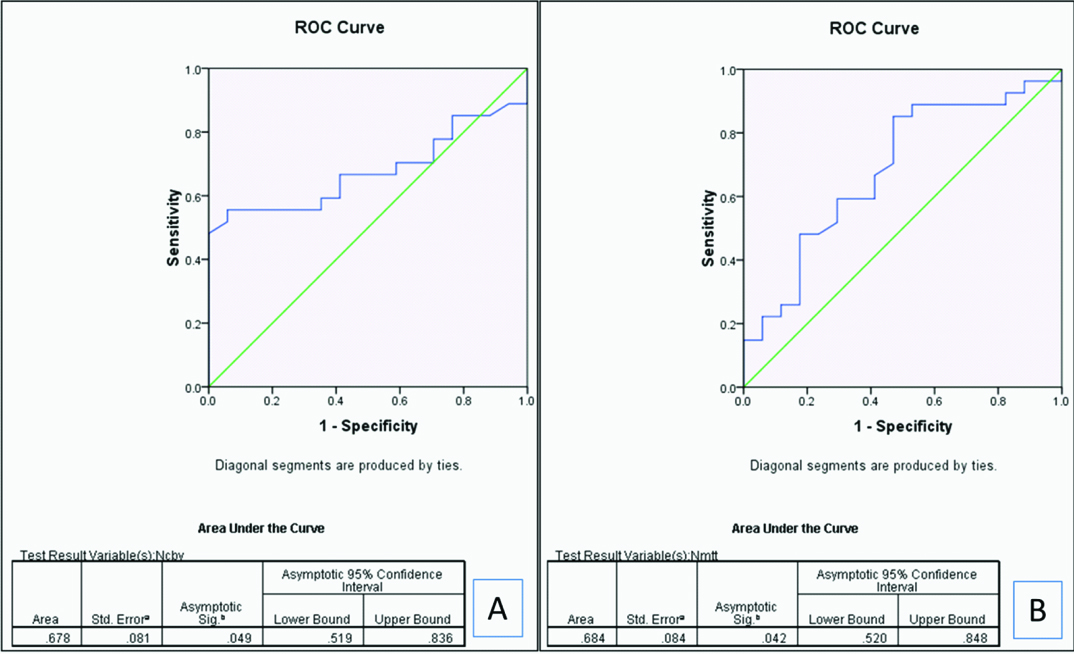
A cut off of > 4.95 for nCBV yielded 94.1% sensitivity and 55.6% specificity and > 1.88 for nMTT yielded 48.1% sensitivity and 82.4% specificity in identifying metastases [Table/Fig-12].
Sensitivity and specificity of mean nCBV and nMTT to identify metastasis.
| Cut off | Sensitivity | Specificity |
|---|
| nCBV* >4.95 | 94.1 % | 70.6% |
| nMTT†>1.88 | 48.1 % | 82.4% |
- normalized Cerebral Blood Volume,
- normalized Mean Transit Time
Statistical comparison of high grade gliomas and abscess: None of the perfusion parameters were statistically significant in differentiating high grade gliomas and abscess [Table/Fig-3].
[Table/Fig-13,14,15,16 and 17] show CT perfusion images, colour maps, time attenuation curves and perfusion parameters values for glioblastoma multiforme, low grade glioma, primary CNS lymphoma, metastases and abscess respectively.
Image [1] CT perfusion image showing histologically proven case of glioblastoma multiforme involving the right frontal lobe and the corpus callosum. Image (2) shows corresponding CBV, CBF, MTT and TTP colour maps of the lesion. Image (3) shows arterial (red), venous (blue), tumoural (yellow) and normal parenchymal (white) time attenuation curves. As compared to the normal parenchyma the lesion shows increased CBV, CBF, MTT & TTP values as seen in the ROI table.
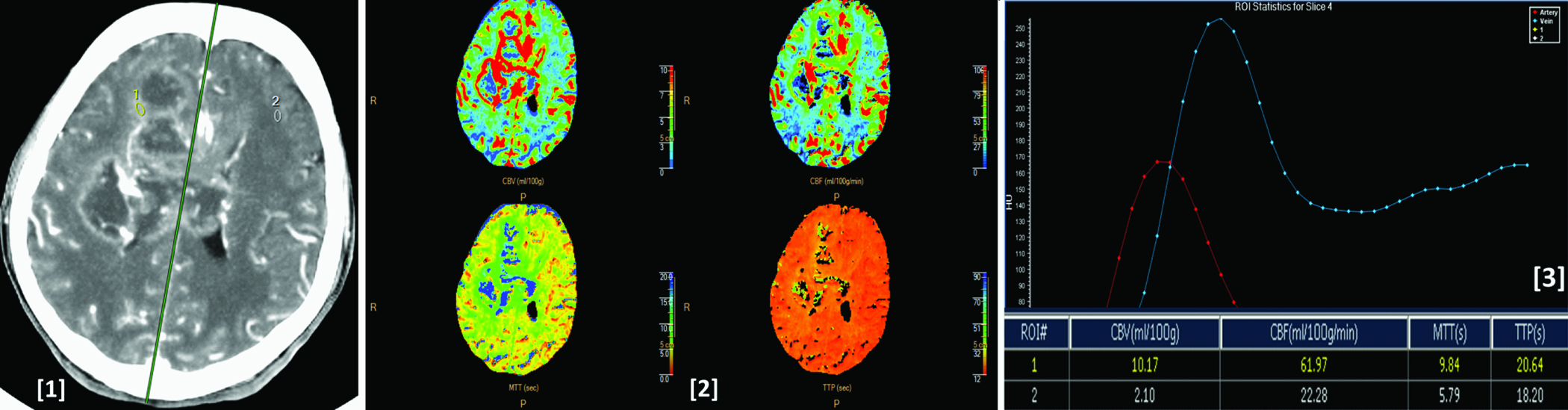
Image (1) shows CT perfusion image showing histologically proven case of low grade glioma in the left frontal lobe. Image (2) shows corresponding CBV, CBF, MTT and TTP colour maps of the lesion. Image (3) shows arterial (red), venous (blue), tumoural (yellow) and normal parenchymal (light blue) time attenuation curves. As compared to the normal parenchyma the lesion shows mild increased CBV decreased CBF, increased MTT and TTP values as seen in the ROI table.
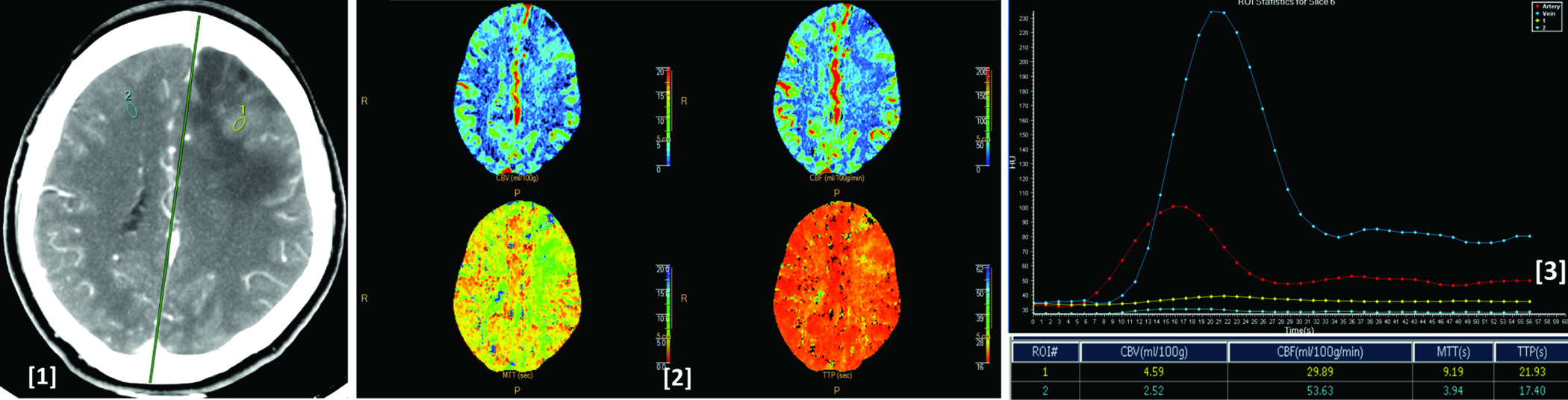
Image (1) shows CT perfusion image showing a histologically proven case of Primary CNS lymphoma in the left thalamus. Image (2) shows corresponding CBV, CBF, MTT and TTP colour maps of the lesion. Image (3) shows arterial (red), venous (blue), tumoural (yellow) and normal parenchymal (light blue) time attenuation curves. As compared to the normal parenchyma the lesion shows increased CBV, CBF, MTT and TTP values as seen in the ROI table.
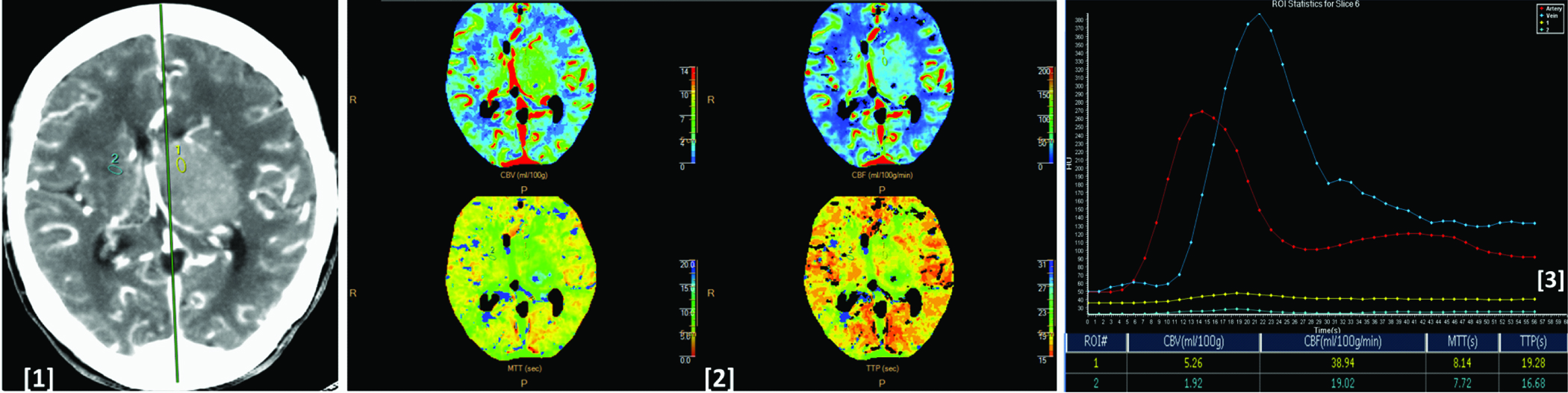
Image (1) shows CT perfusion image showing metastatic lesions in bilateral parietal lobes in a patient with bronchogenic carcinoma. Image (2) shows corresponding CBV, CBF, MTT and TTP colour maps of the lesions. Image (3) shows arterial (red), venous (blue), tumoural (yellow) and normal parenchymal (light blue) time attenuation curves. As compared to the normal parenchyma the lesion shows increased CBV, CBF, MTT and TTP values as seen in the ROI table.
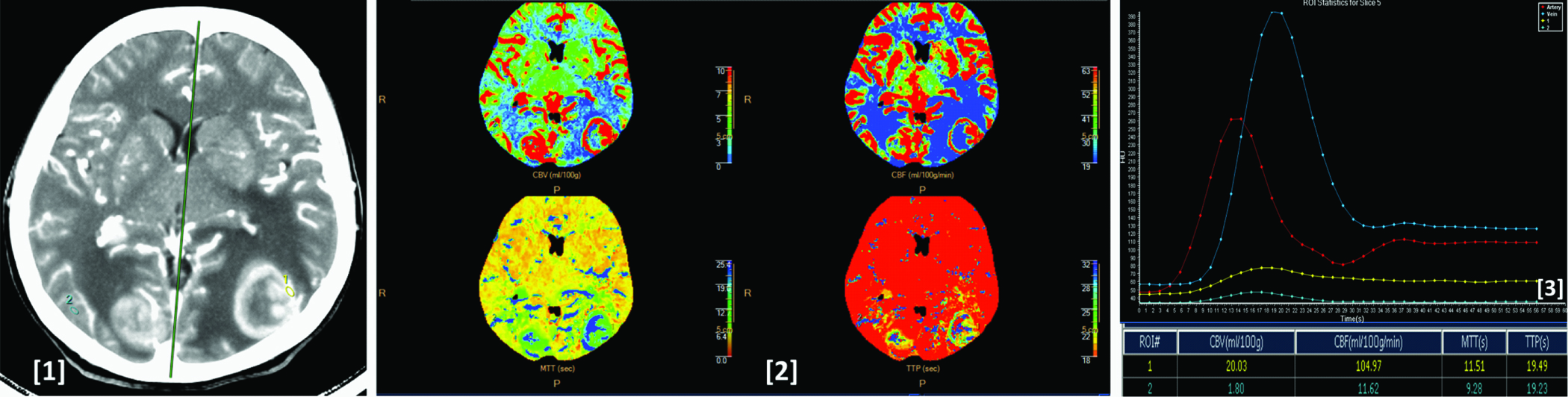
Image (1) shows CT perfusion image showing a proven case of abscess in the posterior fossa. Image (2) shows corresponding CBV, CBF, MTT and TTP colour maps of the lesion. Image (3) shows time attenuation curves of the artery (red), vein (blue), lesion (yellow) and normal parenchymal (light blue). As compared to the normal parenchyma the lesion shows increased CBV, CBF, MTT and TTP values as seen in the ROI table.
Discussion
Vascular proliferation and blood supply directly relates to the contrast enhancement on perfusion weighted images. Knowing the neovascularity and increased microvasculature within the tumour is essential to decide or diagnose its aggressiveness or grade. Being the most frequently encountered tumours, gliomas can be low grade or high grade. Hence, apart from morphological criterias on CT, such grading can also be based on the vascular properties that are derived through dynamic scans [7].
MRI perfusion imaging can also offer such characterization both through contrast and non-contrast dynamic imaging methods apart from having the advantage of no radiation side-effects.
However, MR perfusion can only offer a semi-quantitative perfusion assessment by comparing an abnormal area to a normal area [8]. Apart from the perfusion imaging, MRI also offers diffusion weighted imaging and spectroscopic imaging which can be added to the routine protocol to increase the confidence while characterizing the intracranial lesion.
CT perfusion is simpler as compared to MRI as the attenuation values and the contrast concentration bear a more linear relationship [5] and hence, delivers a “superior quantitative accuracy” by providing absolute quantitative values of the perfusion parameters [8]. Though the cost of the CT imaging may increase by adding CT perfusion imaging to the routine CT protocol, we believe the over-all cost would still be lesser than MR imaging (with MR perfusion added) especially in developing countries.
Ellika SK et al., showed that mean nCBV and nCBF of high grade gliomas are significantly higher as compared to the low grade gliomas with a cut point for nCBV of >1.92 yielding 85.7% sensitivity and 100% specificity, >1.48 of nCBF yielding 71.4% sensitivity and 100% specificity [9]. Jain R et al., also showed similar results [10].
rCBV provided significant p-value in glioma grading in a study conducted by Ding B et al., [11]. Present study shows good correlation with the above studies. nCBV stood the best parameter useful in characterizing the gliomas into high or low grade among all. No statistically significant difference was seen in the mean nMTT among low and high grade gliomas which was similar to studies by Ellika SK et al., and Jain R et al., [9,10]. However, there was a trend for lower nMTT in high-grade gliomas with mean of the parameter being lower in high grade as compared to the low grade gliomas. nTTP also showed no statistically significant difference among high and low grade gliomas.
Primary CNS lymphomas form upto 6% of malignant tumours in CNS [12]. Due to their diffuse infiltrative growth, at times it becomes a diagnostic challenge in deciding it is lymphoma or a glioma [13].
Perfusion CT can help in such situation as the histopathological property of lymphomas in lacking vascular proliferation can be easily detected through dynamic contrast CT imaging [14].
Schramm P et al., [15] showed significantly higher CBV values in high grade gliomas than lymphomas (p= 0.0078) and concluded that based on CBV quantitative measurement, perfusion CT can reliably classify gliomas and lymphomas. Our study shows good correlation to their study with respect to nCBV parameter. However, nMTT stood the best parameter in identifying primary CNS lymphomas by showing higher specificity as compared to nCBV in our study. Mean nCBF of high grade gliomas showed no significant difference as compared to PCNSLs.
The growth of metastatic lesions occurs due to its nature of inducing angioneogenesis and hence, they are generally expected to have increased nCBV. This results in overlap of the perfusion parameters of metastatic lesions with that of high grade gliomas as both the lesions induce angiogenesis [16]. In present study, breast carcinoma (n=9), bronchogenic carcinoma (n=6), renal cell carcinoma (n=5), follicular carcinoma of thyroid (n=3), colorectal carcinoma (n=2), malignant melanoma (n=1) and neuroendocrine tumour of pancreas (n=1) formed the primaries for a total of 27 cases.
Sankhe S et al., in their study found higher nCBV in metastases with a mean of 5.43±2.1 [17]. The present study also showed a higher nCBV mean of 7.33±5.80. The intratumoural mean nCBV and nMTT of metastatic lesions were significantly higher (p<0.05) as compared to the high grade gliomas in our study. Study by Kamble RB et al., showed no significant difference (p>0.05) in the perfusion parameters in characterizing the two conditions [18]. The difference seen between our study and the study by Kamble RB et al., could be due to high sample size of metastases (n=27) in present study as compared to lower sample size (n=6) in their study. It may also be noted in the [Table/Fig-11] (ROC curves) that the asymptotic significance (p-values) for nCBV and nMTT are 0.049 and 0.042 respectively, which are just at the borderline of significant p-value of less than 0.05. Hence, we believe a higher sample size clearly can show a statistically significant difference.
Inspite of the known morphological criterias in differentiating an abscess from a neoplasm with rim enhancement, often the distinction between the two becomes difficult both on CT and MRI.
Infective lesions like tuberculomas, abscess and toxoplasmosis show a reduced rCBV as observed in a different study by Kamble RB et al., [19]. MR perfusion was performed by Holmes TM et al., on four patients having abscess and four patients having high grade gliomas where they found the mean rCBV of high grade gliomas to be significantly higher than the abscess [20]. Our study comprised of 17 cases of high grade glioma and seven cases of abscess and showed no significant difference in mean nCBV among both the conditions. However, the high grade gliomas showed a trend towards higher nCBV as compared to the abscesses as noted in [Table/Fig-2]. Mean nCBF, nMTT and nTTP also showed no significant difference among high grade gliomas and abscess.
Limitation
Limitation of this study was the restricted slice number during acquisition of perfusion images as only 4 cm of tissue of interest could be imaged with the 64 slice CT scanner. Hence, the whole tumour volume could not be imaged in full. Thus, our limited region of interest might have been “nonrepresentative” of whole tumour perfusion, especially in large and heterogeneous lesions.
A relatively small sample size for each of the conditions was another limitation of the study.
Conclusion
CT perfusion can be added on as an adjunct to anatomic imaging to increase the accuracy of CT diagnosis for intracranial lesions. To date CT perfusion is the only technique that has enabled noninvasive absolute quantification of perfusion parameters. The technique potentially has both clinical and research applications in evaluation of brain pathologies.
*- normalized Cerebral Blood Volume,
†- normalized Cerebral Blood Flow,
‡- normalized Mean Transit Time,
§- normalized Time To Peak.
*- normalized Cerebral Blood Volume,
†- normalized Cerebral Blood Flow,
‡- normalized Mean Transit Time,
§- normalized Time To Peak,
||- High Grade Glioma,
**- Low Grade Glioma,
††- Primary CNS Lymphomas
*- normalized Cerebral Blood Volume,
†- normalized Cerebral Blood Flow
*- normalized Cerebral Blood Volume,
†- normalized Cerebral Blood Flow
*- normalized Cerebral Blood Volume,
†- normalized Mean Transit Time