Gastric Glomus Tumour Misdiagnosed as Gastric Carcinoid: An Unfamiliar Entity with Aids to Diagnosis and Review of Literature
Tanush Vig1, Mandeep Singh Bindra2, Ramani Manoj Kumar3, Suceena Alexander4
1 Assistant Professor, Department of Pathology, Christian Medical College and Hospital, Vellore, Tamil Nadu, India.
2 Associate Professor, Department of Pathology, Christian Medical College and Hospital, Vellore, Tamil Nadu, India.
3 Associate Professor, Department of Pathology, Christian Medical College and Hospital, Vellore, Tamil Nadu, India.
4 Professor, Department of Nephrology, Christian Medical College and Hospital, Vellore, Tamil Nadu, India.
NAME, ADDRESS, E-MAIL ID OF THE CORRESPONDING AUTHOR: Dr. Tanush Vig, 4th Floor, Department of Pathology, ASHA Building, Christian Medical College and Hospital, Ida Scudder Road, Vellore, Tamil Nadu, India.
E-mail: medicovig@gmail.com
Glomus tumour (GT) is a rare mesenchymal tumour of the stomach with Gastrointestinal Stromal Tumour (GIST), leiomyoma and schwannoma being far more common and comprising more than 90% of all gastric mesenchymal tumours. As glomus bodies are located in the peripheral parts of the human body, these tumours are peripherally located, classically the subungual region, hands, feet and trunk. While being evaluated for renal problems, a middle aged lady was incidentally found to have a gastric tumour. This was submucosal in location and was excised by a wedge resection and reported elsewhere as carcinoid tumour. The patient came to our hospital for further management. The biopsy was reviewed here and the modified diagnosis given was GT, confirmed by panel of immunohistochemistry. Two years after regular clinical follow up the patient is free of disease or any distant metastasis. In this paper the authors discuss the potential pitfalls, differential diagnoses and diagnostic clues that help in diagnosing this gastric tumour.
Mesenchymal tumour, Neuroendocrine, Stomach, Submucosa
Case Report
A 49-year-old lady was evaluated elsewhere for complaints pertaining to deteriorating renal function. Routine clinical and laboratory examination revealed non-functioning right kidney with Grade-IV hydroureteronephrosis. During the abdominal ultrasound scan, an incidental gastric lesion, 4x4 cm in the submucosal plane was also identified. An Octreotide-PET scan was done later which revealed increased uptake in the gastric lesion, but showed no evidence of metastatic disease. She subsequently underwent laparoscopic right nephrectomy and wedge resection of the gastric tumour. The histopathological report on the kidney confirmed chronic pyelonephritis and the gastric tumour was reported as well-differentiated neuroendocrine tumour with a MIB-1 proliferation index of 2%-3%. The patient came to our tertiary-level centre with paraffin embedded blocks for confirmation of diagnosis and further management of her disease.
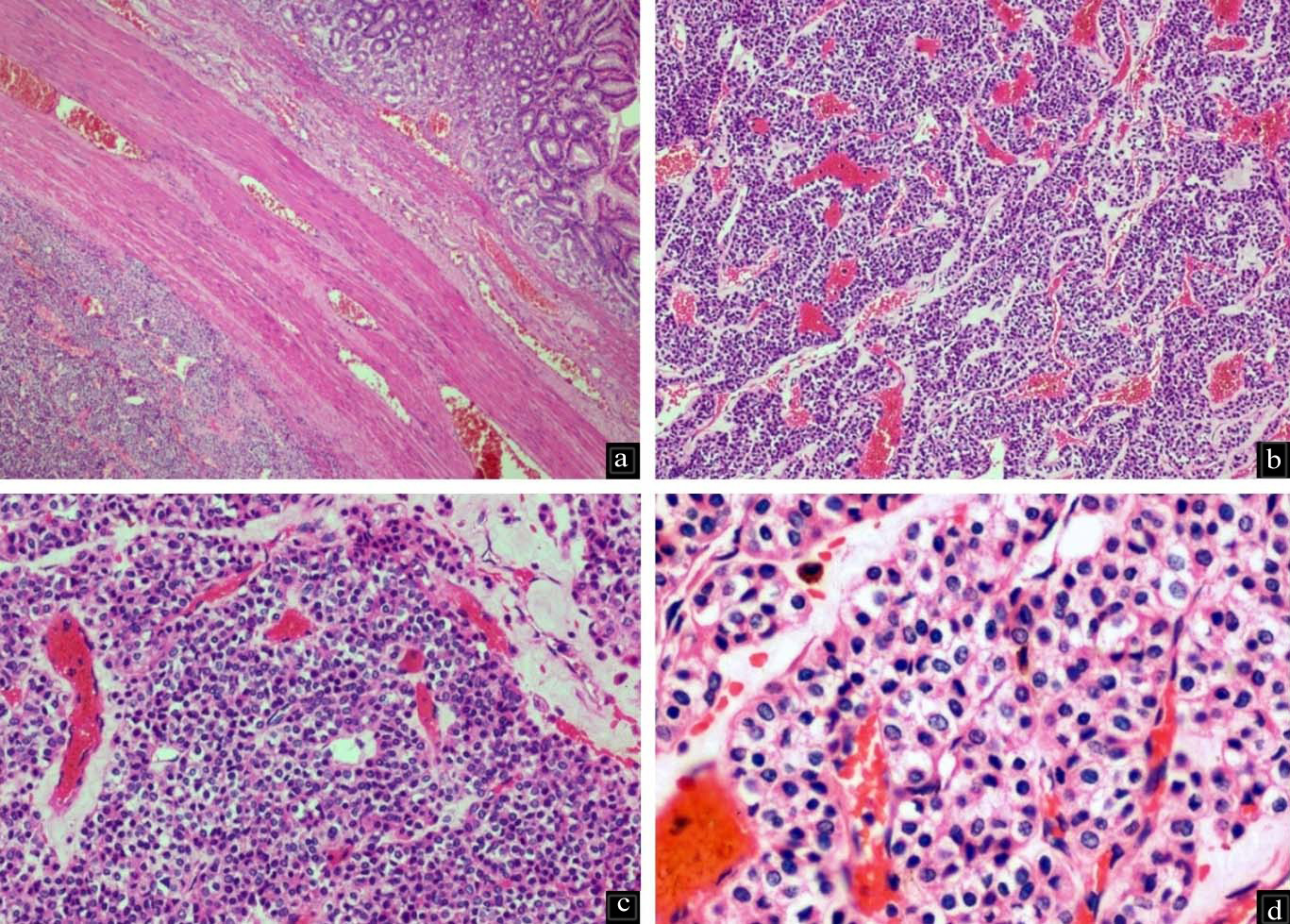
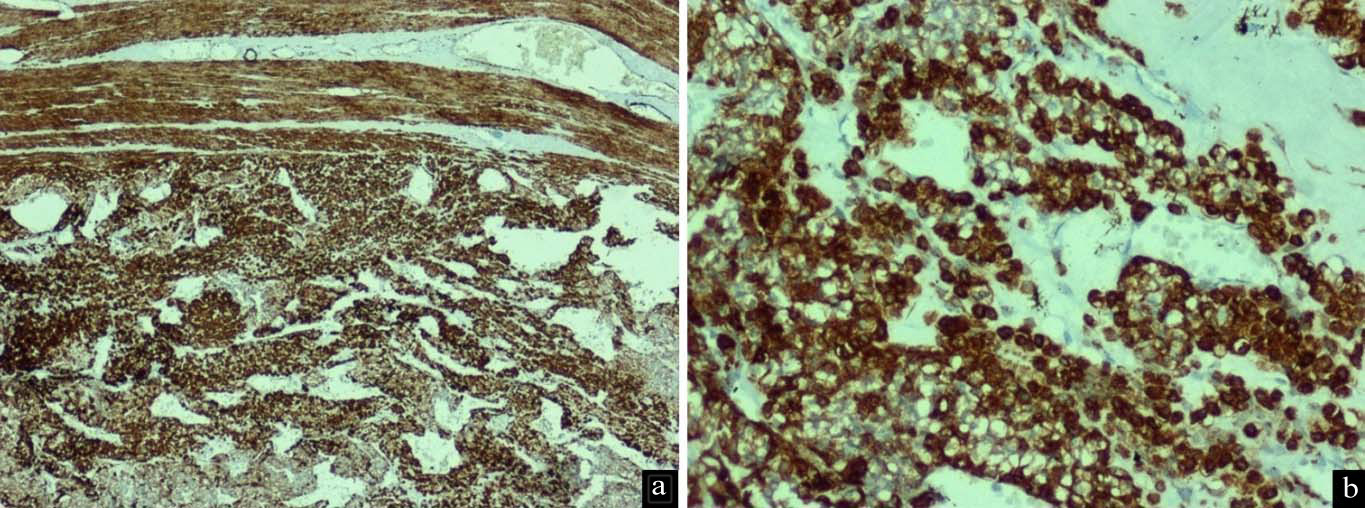
Histopathological examination of the submitted paraffin blocks taken from the gastric tumour showed wall of gastric antrum with a tumour within the subserosal connective tissue arranged in nests and anastomosing trabeculae invested by delicate, congested vascular channels [Table/Fig-1a-c]. The tumour cells were round and monotonous with well defined borders exhibiting round to oval nuclei with inconspicuous nucleoli. There was no mitosis, cyto-morphological atypia or necrosis and the overlying gastric antral mucosa was normal [Table/Fig-1d]. In view of the referring diagnosis of neuroendocrine tumour, markers to support the same were done, but found to be negative (Synaptophysin, Chromogranin, CD56 and Pancytokeratin). GIST was ruled out by CD117 negativity. The morphological features were suspicious of GT and subsequent diffuse positivity for SMA confirmed the diagnosis [Table/Fig-2a,b]. Thus, a final diagnosis of gastric GT was given.
a) Wall of stomach with tumour in the muscularis propria, bottom-left of the image H&E 4X); b,c) Tumour is arranged in nests and trabeculae traversed by ramifying and congested vascular septae (H&E 10X and 20X); d) Tumour cells are monotonous with minimal nuclear pleomorphism and moderate amounts of pale eosinophilic cytoplasm. Occasional cells with clear cytoplasm are also present (H&E 40X).
Tumour cells show diffuse strong positivity for Smooth Muscle Actin - SMA IHC: a) 10X and; b) 20X.
Perhaps the reason for misdiagnosis as a neuroendocrine tumour was the morphological overlap and possible lack of detailed immunohistochemical examination. This was a block review and original slides were not available, the authors assume that the diagnosis of neuroendocrine tumour was made purely on histology and MIB-1 was performed for grading as per the WHO classification. Patient is on regular follow-up and 24 months post surgery has had no recurrence or any metastatic event.
Discussion
Glomus Tumours (GT) are the neoplastic counterpart of perivascular glomus bodies that are located in the extremities, principally involved in core-temperature regulation and homeostasis consisting of intricate arterio-venous shunt (the Sucquet-Hoyer canal) [1,2]. They are most numerous in the fingers and toes, hence GT is most commonly found in the subungual location. These lesions are relatively common and occur most commonly in young adults with no gender predilection. Apart from the peripheral soft tissues other sites reported to be involved are the osteoarticular system, muscle, tympanum, mediastinum, trachea, lung, kidney, uterus and vagina. Visceral involvement is extremely rare as glomus bodies are virtually non-existent and only a limited number of cases have been reported in the English literature [1–5]. Gastric GT was first reported by Kay S et al., in 1951 and till date less than 100 cases have surfaced thereafter in the English literature as they are greatly outnumbered in frequency by other mesenchymal gastric tumours, most commonly GISTs [3].
Miettinen M et al., reported the largest case series and observed gastric GTs to be 100 times rarer than gastric GISTs [2]. These tumours show a female sex predilection and occur more commonly in the fifth or sixth decade, but a wide range of age has been encountered [2,4]. Being mesenchymal tumours they are usually submucosal in location occurring as an asymptomatic intraluminal growth or as a mass lesion on the serosa. They can occasionally be symptomatic causing gastrointestinal bleeding, malaena and anaemia [5]. The vast majority of gastric GTs are benign, and the malignant form is exceedingly rare, with only a handful of cases documented in the literature [6,7].
Highlight of the case presented herein is two-fold. First is the rarity of GT in the stomach that practising pathologists and gastroenterologists may hardly ever come across in their vast career spanning decades. Second is the morphological overlap this neoplasm has with a far more commonly encountered neuroendocrine tumour that leads to diagnostic pitfall.
Macroscopically the tumour is seen as a polypoidal lesion with intact overlying mucosa or a bulging nodularity on the visceral surface. Currently available imaging studies like CT and MRI have been found to be unreliable in accurately differentiating GT from other lesion like GIST, leiomyomas, neuroendocrine tumours or ectopic pancreas. Invasive procedure like the Endoscopic Ultrasound (EUS) is also not sufficient to diagnose GT [4], with exceptions [8]. EUS-guided aspiration combining cytological examination and immunohistochemistry has also recently been described to help in diagnosis, however due to the dependency on aspirate yield and rich vascularity of the tumour, this technique has limitations and potential complication risks. As a result the diagnosis of GTs rests on histopathological examination and immunohistochemistry.
The morphology of visceral GT is similar to those in their usual locations with a monotonous population of round cells devoid of atypia. Tumour cells are immunopositive for mesenchymal markers like α-Smooth Muscle Actin (SMA), laminin, collagen type IV, and vimentin. MIB-1 proliferation index is low, usually 1%-2%. Recent studies have also identified the presence of BRAF mutations in some GTs [9]. CD34 can be focally positive in a few gastric GT, however the peripheral GT are more commonly positive for CD34 [2].
S-100 protein, CD31, HMB-45, Melan-A, cytokeratin, CD56, CD117 and chromogranin are negative [2,4,10,11]. Occasional cases with clear cell change may show weak positivity for synaptophysin. Histological differential diagnoses can include carcinoid tumour, epithelioid GIST, paraganglioma and low grade lymphoma. GIST is the most common mesenchymal tumour of GI tract with an epithelioid variant posing diagnostic dilemma. GISTs are diffusely positive for CD117 (c-kit) and DOG-1 whereas GTs are consistently negative. Paragangliomas have a characteristic organoid (insular) pattern separated by thin-calibre vessels, tumour cells displaying nucleomegaly, hyperchromasia and moderate amount of eosinophilic cytoplasm. They are immunopositive for synaptophysin, chromogranin, S-100 (sustentecular cells) and immunonegative for SMA.
The closest morphological mimicker is a well-differentiated grade 1, neuroendocrine tumour (carcinoid) that grows in nests and trabeculae of oval to polygonal cells invested by thin calibre vessels without significant atypia, mitosis or necrosis. Carcinoid/NET is positive for synaptophysin, chromogranin, cytokeratin, CD56 and NSE with consistent immunonegativity for SMA and CD34.
Gastric GT are benign in biological behaviour. However, a few may metastasize and a strict criterion has not been established as tumours with minimal mitotic activity (1-3/50 HPF) have also metastasized. Rare cutaneous metastasis from gastric GT are also on record [7]. Criteria for malignant GT has been proposed by the study of Folpe AL et al., [6].
As majority of gastric GT are clinically benign, wedge resection with an adequate tumour-free margin is currently the treatment of choice [1]. The rarity of this tumour is reinforced by the paucity of published literature, most of which are case reports and occasional case studies.
Conclusion
Through this publication, we wish to generate awareness about this rare mesenchymal tumour among practising gastroenterologists and pathologists. Pathologists should entertain a wider list of differential diagnoses, be aware of potential diagnostic pitfalls and ultimately clinch the diagnosis of gastric GT with the help of immunohistochemistry.
[1]. Tsuneyoshi M, Enjoji M, Glomus tumour: a clinicopathologic and electron microscopic studyCancer 1982 50(8):1601-07. [Google Scholar]
[2]. Miettinen M, Paal E, Lasota J, Sobin LH, Gastrointestinal glomus tumours: a clinicopathologic, immunohistochemical, and molecular genetic study of 32 casesAm J Surg Pathol 2002 26(3):301-11. [Google Scholar]
[3]. Kay S, Callahan WP, Murray MR, Randall HT, Stout AP, Glomus tumours of the stomachCancer 1951 4(4):726-36. [Google Scholar]
[4]. Kang G, Park HJ, Kim JY, Choi D, Min BH, Lee JH, Glomus tumour of the stomach: a clinicopathologic analysis of 10 cases and review of the literatureGut Liver 2012 6(1):52-57. [Google Scholar]
[5]. Diaz-Zorrilla C, Grube-Pagola P, Remes-Troche JM, Medina AR-D la, Glomus tumour of the stomach: an unusual cause of gastrointestinal bleedingBMJ Case Rep 2012 2012:bcr2012007391 [Google Scholar]
[6]. Folpe AL, Fanburg-Smith JC, Miettinen M, Weiss SW, Atypical and malignant glomus tumours: analysis of 52 cases, with a proposal for the reclassification of glomus tumoursAm J Surg Pathol 2001 25(1):1-12. [Google Scholar]
[7]. Bray APJJ, Wong NACS, Narayan S, Cutaneous metastasis from gastric glomus tumourClin Exp Dermatol 2009 34(8):e719-21. [Google Scholar]
[8]. Gu M, Nguyen PT, Cao S, Lin F, Diagnosis of gastric glomus tumour by endoscopic ultrasound-guided fine needle aspiration biopsy. A case report with cytologic, histologic and immunohistochemical studiesActa Cytol 2002 46(3):560-66. [Google Scholar]
[9]. Chakrapani A, Warrick A, Nelson D, Beadling C, Corless CL, BRAF and KRAS mutations in sporadic glomus tumoursAm J Dermatopathol 2012 34(5):533-35. [Google Scholar]
[10]. Lee H-W, Lee JJ, Yang DH, Lee BH, A clinicopathologic study of glomus tumour of the stomachJ Clin Gastroenterol 2006 40(8):717-20. [Google Scholar]
[11]. Wang Z-B, Yuan J, Shi H-Y, Features of gastric glomus tumour: a clinicopathologic, immunohistochemical and molecular retrospective studyInt J Clin Exp Pathol 2014 7(4):1438-48. [Google Scholar]