Tencate defined eruption as “the movement of teeth from its developmental position within the bone to its functional position in the occlusion” [1]. For the study purpose, most of the investigators consider a tooth as erupted if tooth with any part of its crown penetrates the gingiva and is visible in the oral cavity [2-4]. Primary tooth development starts at 14 weeks in-utero and is fully completed by 11 months in post natal life [5,6].
Eruption of first primary teeth is the most anxiously awaited developmental milestone in a child and achieving this milestone by a child gives a great pleasure and satisfaction to parents. Factors like ethnic origin, nutritional status, socio economic status vary from one geographic region to another, which might affect the timing of tooth eruption. Hence, it may be recommended to have population specific references [7-12]. This has led the researchers all over the world to publish standard of tooth emergence specific to the population or ethnic group [7-11].
Timing of primary teeth is represented as mean or median age of eruption with its normal standard deviation and percentile range. Mean or median alone are insufficient for prediction of tooth eruption sequence because they provide no information on the frequency of sequence variation within the pairs of teeth [13]. Eruption sequence can be a very valuable asset in diagnosis and treatment planning during growth and development of child [14].
In this regard, norms of polymorphic variation in the eruption sequence can be more useful. When such norms are available a clinician can assess the normality of a given sequence of teeth eruption in a single child. For e.g., for a child whose mandibular canine is erupted before mandibular molar; knowing that such a sequence is rare (prevalence <5%) [15], in the population to which the child belongs, enables the paediatrician/pedodontist to judge how much normal that sequence is and helps in planning the treatment. Scientific literature has showed a very few published article related to polymorphism in the sequence of eruption. Through Pubmed search we could retrieve only one article describing the polymorphism in eruption sequence of primary teeth [15]. Hence, the present study aimed at providing norms for the sequence polymorphism in primary teeth among the children of Mysore population.
Materials and Methods
A cross-sectional study was designed with a total of 1392 children (778 males, 614 females) aged 03-36 months, who visited the Immunization Center and Outpatient Clinic of Department of Paediatrics, JSS Hospital, Mysore, Karnataka, India, from December 2015 to June 2016 were recruited by simple random sampling method. In order to reduce the ethnical divergence, which might influence the results, children of the parents who are Mysore citizens and have been living in Mysore for more than three generation were included in the study. Other criteria for inclusion were, children who were born at term or >37 completed weeks of gestation, children with birth weight 2.5-3.2 kg and children from same socio economic status (Kuppuswamy socio economic status class III). Children with genetic diseases, chronic infectious systemic diseases, nutritional and endocrinal disturbances and with recognized syndromes and developmental disturbances like cleft lip and palate were excluded from the study since these factors causes variation in the eruption timing. Chronological age in months was calculated for every child from hospital records/ birth certificates. The study was approved from Institutional Ethical and Review Committee. Written informed consent was obtained from all the parents of the study subjects.
Principle investigator was calibrated in pilot study consisting of 30 subjects. All the subjects were checked twice at an interval of six days between the first and second visit. Later these subjects were excluded from the main study. Clinical examination of the subject was done under natural illumination using disposable mouth mirror and tongue depressor using lap to lap position on an ordinary chair. A tooth was considered erupted when any part of its crown has penetrated the gingiva and was visible in the oral cavity [16].
Statistical Analysis
Intra examiner reliability was evaluated using two sample paired student’s t-test. Using the statistical package SPSS 22.0 data processing and analysis was carried out. Many studies have shown insignificant difference in the timing of primary eruption across the sides, which made the investigators to report the data for the right side [2-4,15]. Following the same approach results of the right side is presented.
Across the entire possible intra quadrant tooth pair cases of present-present, absent-absent, present-absent and absent-present were counted and computed. The counts related to present-present and absent-absent were excluded because they would give no information on eruption sequence in a cross-sectional study. For e.g., let us consider tooth pair of lateral incisor and first molar, if both lateral incisor and first molar are present at the time of examination, it is not possible to assume which erupted first, similarly when they are absent; one cannot predict which will erupt first.
For pair wise counts related to present-absent and absent-present, the frequencies of the sequence polymorphism were calculated and expressed as fractions/percentages. The numerator was either present-absent or absent-present and denominator was the sum of present-absent and absent-present for a given intra quadrant pair of teeth [17]. Following the method adapted by Smith and Gran, the frequencies of sequence polymorphism were rounded to the nearest whole percentage [13]. Frequencies <5% were considered rare and above 5% were polymorphic. The limit 5% was chosen because any frequency equal to or more than 5% should be significantly different from zero at p-value of 0.05 [13].
Results
Student’s t-test was applied to check the intra examiner reliability which showed an insignificant difference in the dental examination outcome between the first and the second visit (p <0.05).
All the teeth are represented in FDI notation. Sequence polymorphisms in the eruption of primary teeth are represented in four matrix describing pair wise frequency separated by gender and arch [Table/Fig-1]. The presentation of the matrix form follows the approach used in the similar study on Jordanian population [15]. In each matrix the teeth are order in the sequence reported by Gunashekar M et al., [18].
Matrices of pair wise frequencies (%) of erruption sequences separated by arch and gender. Teeth listed vertically are ‘present’ and teeth listed horizontally are; absent’. Total numbers of present-absent and absent-present occurrences are given above the diagonal in parenthesis. Teeth are represented in FDI system.
A-Absent, P-Present.
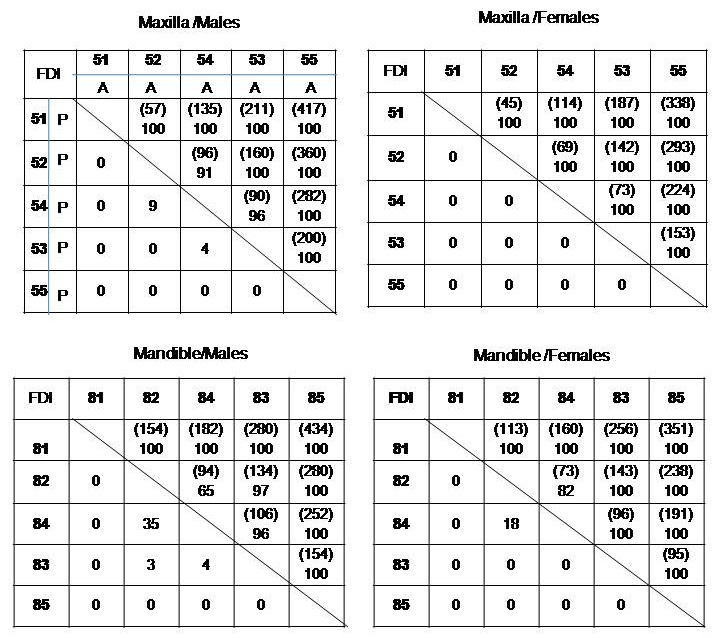
In the matrices, teeth listed horizontally are ‘absent’ and teeth listed vertically are ‘present’. Therefore, below the diagonal are the frequencies of present-absent and above the diagonal are absent-present frequencies. The total numbers of present-absent and absent-present occurrences for each pair are given above the diagonal in the parenthesis. Present-absent frequencies listed below the diagonal designate less common occurrence (reverse sequence).
As in the [Table/Fig-1], Maxilla/Males matrix, teeth represented horizontally are absent and teeth represented vertically are present. If we consider 52-54 pair of teeth, below the diagonal, 54 is present and 52 is absent, this occurs in 9% of the population. Whereas above the diagonal 54 is absent and 52 is present, this occurs in 91% of the population. The number in the parenthesis represent the total present absent and absent present occurrences. In our study out of 778 males, 52 was present in 430 subjects and 54 was present in 352 subjects. However, the tooth pairs ‘52 present-54 absent’ was in 87 subjects and ‘54 present-52 absent’ was in nine subjects. So the total numbers of present-absent and absent-present occurrences for 52-54 tooth pair are 96(87+9) given above the diagonal in the parenthesis. This total will be the frequency denominator to calculate the above percentages. Likewise the percentages were calculated for all the other matrices in the [Table/Fig-1].
From [Table/Fig-1], it is evident that tooth pair which exhibits significant polymorphic reverse sequence was 52-54 (9%), 82-84 (35%) in males and 82-84 (18%) in females. The highest polymorphic frequencies were in the mandibular arch in 82-84 (35% in males and 18% in females). Thus generally sequence polymorphisms were more common in 82-84 pairs of teeth. In females there was no polymorphism in maxillary arch. Compared to teeth in maxillary arch mandibular teeth showed more sequence polymorphism in both males and females.
Discussion
In the present study, children aged 3-36 months were selected. All the children were almost equally distributed among all the age group. Since children of this age visit the paediatrician more frequently for immunization and well baby check up, hospital based recruitment of the study subjects was more appropriate. All the subjects were selected from single hospital since this is one of the biggest hospitals in Mysore with the highest number of children visiting the Paediatric OPD. For the present study, children belonging to the same social economic status were selected to eliminate the effect of socio economic status on eruption.
Clinical examination of subjects was done without the radiographs. Hence, the teeth recorded as absent (unerupted) might be congenitally missing. However, such a bias has not played a significant role because relatively large sample size reduced the potential effect of those few misidentification if any and secondly the prevalence of hypodontia among the Indian children is low (0.88%) [19]. Also, to minimize the effect of error occurring during the data entry it was ascertained that all the data was entered by single investigator. To control any further error, the eruption sequence frequencies were adjusted to nearest whole percentages. This would to reduce the chance of possible misidentification error which might be present as rare frequency [13,15,17].
Sequence of eruption can be presented in more than one approach as explained by Shawneesh AI [17]. The simplest approach is putting means / medians of tooth in ascending order which most of the studies present. This model provides no information on frequencies of sequence variability [13,20]. They do not provide evaluation and prediction of tooth eruption sequence in individual cases, for e.g., in a population knowing mean/median ages of eruption of teeth, sequence of eruption can be derived. If 82-84 is the sequence derived from median/mean age of eruption in a population, it is inferred that the frequency of reverse sequence i.e., 84-82 is zero. This will mislead the clinician when a single child is evaluated with the sequence 84-82 and may consider it as abnormal. Learning that the frequency of 84-82 was 18% means that it is not uncommon for this sequence to occur among children of that population. On the other hand, if frequency of 84-82 is 2% it means it is a rare occurrence.
The second approach is the one used in present study was based on pair wise frequency in the form of matrices. This overcomes the limitation of providing tooth eruption sequence in order of median/ mean eruption ages. Data provided can be efficiently used for the evaluation and prediction of variation in eruption sequence in individual child. Limitation of this approach is that it provides bidirectional (present-absent; absent present) variations between two teeth at a time without considering sequence variation among all the other teeth in a quadrant. However, this approach is most suitable for cross-sectional study design [17]. The third approach is expressing the variation in eruption sequence across all the intra quadrant teeth instead of pair wise variation. This is most suitable for a longitudinal study design [20].
No published data on sequence polymorphism of primary teeth for Indian children have been reported. Hence, the comparison was made with the only published data on sequence polymorphism of primary dentition on Jordanian children [15]. Sequence variation is expressed in the form of pair wise matrix, the one used in present study. From the present study, it is clear that reverse polymorphism sequence occur when first molar erupts before lateral incisor. Such reverse sequence is more frequent in mandibular arch (35%) than in maxillary arch (9%). These results are consistent with those reported for Jordanian children where the frequency was 14% for mandibular arch and 5% for maxillary arch [15]. This sequence does not seem to present problem in primary occlusion. However, the effect of reverse sequence on permanent dention needs to be investigated. Polymorphism was only in 82-84 tooth pairs both in males (35%) and females (18%), however in maxillary arch females did not show any variation in the sequence of primary teeth eruption, where as Jordanian females showed 5% variation in 54-52 tooth pairs [15].
Limitation
The study was done on one particular population with limited sample size. A large cross-sectional study or a well designed longitudinal study across different population groups in India would give more conclusive results.
Conclusion
To the best of investigators knowledge, there are no previous studies describing the sequence polymorphism in primary teeth in Indian population. Hence, this study provides the basic standard for sequence variation in primary teeth eruption. The data provides evaluation and prediction of tooth eruption sequence in individual child. The results will be valuable in assessment of eruption sequence problems in paediatric dentistry.