Xanthogranulomatous Cholecystitis (XGC) is an uncommon inflammatory condition of gall bladder, which is often misdiagnosed as malignancy preoperatively, leading to extensive surgical resections which may not be necessary for the patient. Ducts of Luschka are a rare developmental variant of the biliary tree, which are prone to injury and bile leak during cholecystectomy. We report a case of a 52-year-old male patient who was taken up for surgery with a provisional diagnosis of chronic calculous cholecystitis. Intraoperative finding of dense adhesions, made the surgeons suspect malignancy. On histopathological examination, it was not only diagnosed as a case of XCG, but it also had florid ducts of Luschka, another rare variant needing documentation as it is a close mimicker of malignancy.
Adhesions, Biliary, Calculous, Gall bladder carcinoma
Case Report
A 52-year-old male presented to the Emergency Department of our hospital with complaints of severe pain abdomen in the right hypochondrium and vomiting since two days. The patient also gave history of similar complaints in the past. On clinical examination, there was right hypochondriac tenderness with Murphy’s sign being positive. His old ultrasound reports were suggestive of calculus cholecystitis. A provisional diagnosis of chronic calculous cholecystitis was made.
Routine blood and biochemical investigations were as follows. Haemoglobin - 11.4g%, Total Leukocyte count –15,800 cells/ cumm, Neutrophils- 88%, Lymphocytes -10% and Eosinophils -2%. Random blood glucose was 120 gm/dl. Total bilirubin was 3.2 g/dl, Direct bilirubin was 2.0 g/dl, Alkaline Phosphatase – 353 IU/L SGOT – 86 IU/L SGPT - 57 IU/L.
Ultrasound abdomen showed multiple calculi of varying sizes, the largest being 2 x 1 cm. There was diffuse wall thickening of the gall bladder thickness (9 mm). With a diagnosis of chronic calculous cholecystitis, the patient was posted for laparoscopic cholecystectomy.
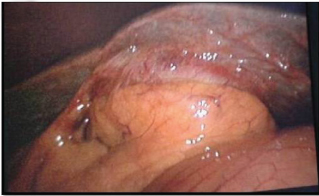
Intraoperative findings were as follows: The omentum was densely adherent to the under surface of the liver, covering the gall bladder. There were dense adhesions from the fundus of the gall bladder to the Calot’s triangle [Table/Fig-1]. Due to difficulty of intraoperative laparoscopic dissection, the procedure was converted to an open one and a retrograde technique was used. The indurated gall bladder along, with dense adhesions was suspicious of malignancy.
Intraoperative picture showing dense adhesions of the gall bladder to the undersurface of the liver.
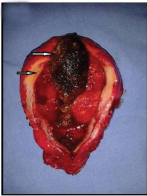
The resected gall bladder was sent for histopathological examination in 10% formalin [Table/Fig-2]. On gross examination, the gall bladder was enlarged, measuring 8 x 4 x 3 cms, serosal surface being congested. Cut section showed a single large mixed cholesterol stone measuring 2 x 1 cm across with multiple small calculi. The mucosa had lost the velvety appearance, and was focally congested. The wall was uniformly thickened (8 mm), was firm in consistency with few bright yellow areas.
Resected gall bladder, cut open with thickened wall (lower arrow) and a large mixed stone (upper arrow). Mucosa appears congested. (All Image left to right)
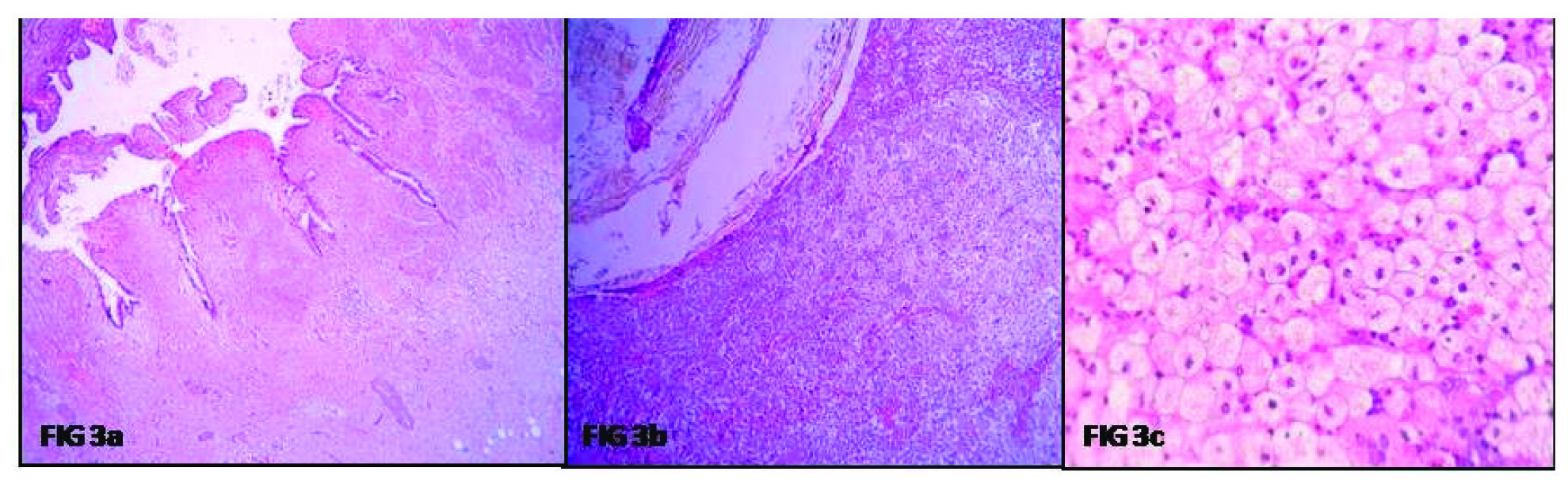
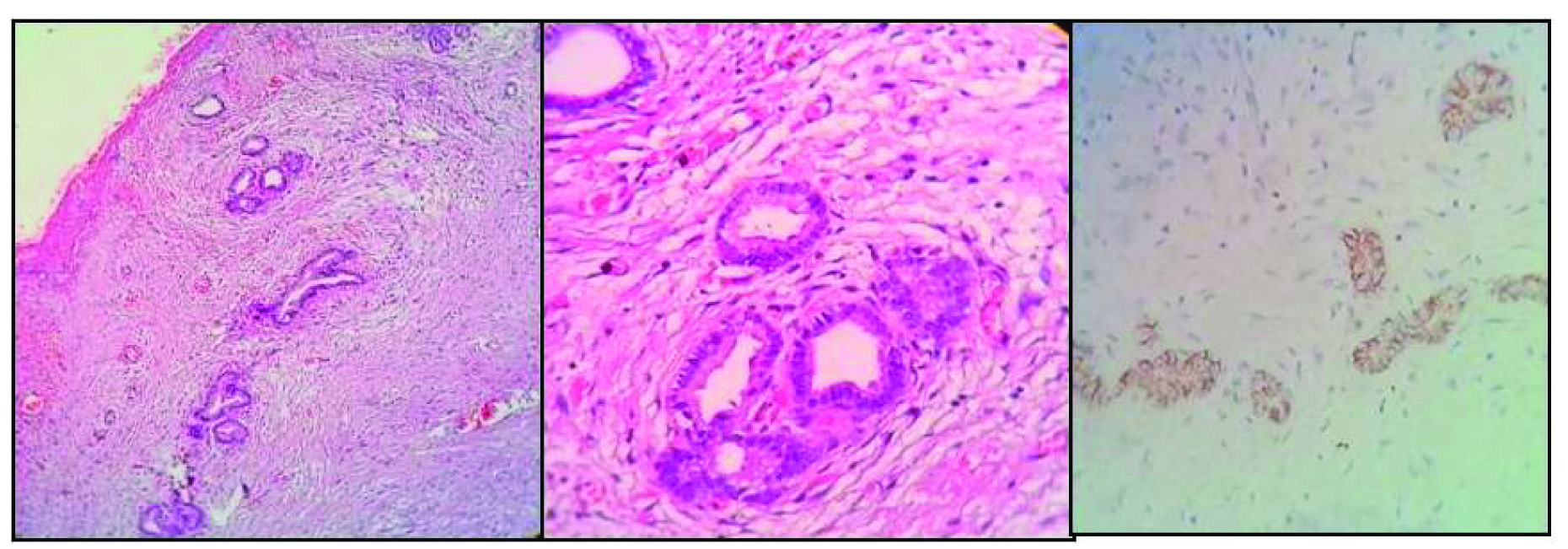
Microscopy showed focally denuded and focally hyperplastic tall columnar lining of the gall bladder. There was extensive surface ulceration and the muscular wall was infiltrated by sheets of foamy histiocytes and cyst macrophages extending up to the serosa. Also, admixed with lymphocytes, plasma cells, foreign body type giant cells, areas of fibrosis and cholesterol clefts [Table/Fig-3a-c]. Few bile plugs were seen. In addition, the serosal aspect showed clusters of tiny ductules, lined by biliary type of epithelium. These were surrounded by dense, concentric areas of fibrosis. However, there were no mitotic figures, nuclear atypia, and irregularity of the glands or foci of such gland like structures, in any other layer of the gall bladder. CD 56 was positive indicating the benign nature of the ducts. A final diagnosis of XGC with florid ducts of Lusckha was conferred upon [Table/Fig-4a-c].
Microphotograph showing a) Gall bladder with focally denuded mucosal lining (H&E 10X); b) Lumen showing bile, probably from the impacted gall stone, and wall showing diffuse infiltration by sheets of foamy cells (H&E 10X); c) Sheets of histiocytes, showing foamy cytoplasm and mild intervening lymphocytic infiltrate (H&E 40X).
Microphotograph showing: a) adventitial layer showing tiny ductules surrounded by concentric fibrosis (H&E 10X); b) closer look at the ductules showing an aggregate of benign ducts lined by biliary type of epithelium without luminal bile (H&E 40X); c) Immunohistochemistry being positive CD 56 in the ductules (H&E 40X).
Postoperative course of the patient was uneventful, after which she was discharged. The patient returned to us for follow up after two months, without any complaints.
Discussion
It is a well-documented fact that inflammatory disease is by far the most common pathology of the gall bladder [1]. Mass forming XGC can mimic gall bladder cancer, making preoperative and intraoperative distinction difficult [2]. Patients are mostly females, with predominant symptoms of vomiting and right upper quadrant pain [3].
Patients with XGC usually exhibit one or more episodes of acute cholecystitis prior to an average of six months of presentation. A past acute cholecystitis episode can be a predictor for diagnosing XGC as it is reflective of the course of the disease [4]. On asking for past history the patient in our case had similar findings.
Ultrasound films almost always show focal or diffusely thickened gall bladder wall with stones, if any, these findings being similar to any case of chronic cholecystitis. On CT the presence of diffuse wall thickening, a large area of the thickened wall being occupied by intramural nodules, absence of intra hepatic invasion, continuous mucosal lines are features favouring XGC over gall bladder cancer [5]. The same was also proven by studies of Goshima S et al., who evaluated CT features of XGC in comparison with gall bladder cancer [6]. However, CT may not be asked for in all cases, considering the need and affordability of the patients, especially who come from a rural area like ours.
The role of XGC as a precursor lesion to malignancy has not been established till date. One study suggests the malignant potential of XGC for its up regulated oncogenes (BCL-2, cMyc), while many studies suggest the pure inflammatory nature of the infiltrate through expression of p53, Proliferating Cell Nuclear Antigen (PCNA) and beta catenin [7].
Intraoperative frozen section is against the en-bloc surgical principal and has the potential to cause tumour cell spreading. Misdiagnosis of this condition comes with a price of extended surgical treatments which can be detrimental to health and recovery of patients [4]. Hong YU et al., in their study concluded that tumour biomarkers like CEA, CA 19-9 and CEA cannot help surgeons make the right diagnosis of XGC [8]. In actuality in many cases it leads to an erroneous misdiagnosis of gall bladder cancer. One of the postulated reasons for elevation in CA–19–9 in XGC is presence of gall bladder stones [8]. A radical resection of the mass is preferred in cases of XGC with extensive infiltration. This is done not only to rule out associated malignancy if any but also to treat symptoms like jaundice and prevent complications like bowel obstruction and perforation [3,8].
Postoperatively, role of the pathologist is not only to confirm the diagnosis of the suspected pathology but also to search for incidental carcinoma. XGC exhibits a focal yellowish hue of the wall in contrast to the homogenous chalky white colour of the carcinomas on gross [1]. Microscopically the tissue in XGC shows, showing diffuse infiltration by foamy histiocytes, surrounding bile plugs, cholesterol clefts, fibrosis, metaplastic epithelium and dense lymphoplasmacytic infiltrate [3].
Luschkas ducts, also called as accessory bile ducts are a developmental abnormality found within the gallbladder fossa in about 10% of the surgically resected gall bladders. They are usually identified in the adventitial layer of the gall bladder wall, without any involvement of the muscular wall, lamina propria or mucosa. They are typically composed of lobular aggregates of small ductules lined by cuboidal to columnar epithelium, surrounded by concentric fibrosis. The lumens lack bile and are usually empty [9]. Singi AD et al., in their study on Lusckas ducts have come across cases of atypical ductules with vesicular nuclei, showing prominent nucleoli and loss of concentric fibrosis, leading to an erroneous diagnosis of malignancy [10]. The ductules in our case were also in the adventitial layer, with concentric fibrosis surrounding them, and were lined by epithelium with bland nuclear features. The differentials to be considered are adenomyomatosis and Rokitansky Ascoff sinuses, both of which communicate through the gall bladder muscular with the lumen and are not restricted to the subserosa. Also, these lesions are usually not surrounded by concentric fibrosis [9,11]. The other differential is adenocarcinoma of the gall bladder, identified by the haphazard infiltrative growth pattern, incomplete glands, mitotic figures and desmoplasia [10]. However, we still went ahead with markers, to confirm the benign nature of the ductules, as this was the first of such cases in our experience. The ducts were positive for CD 56 confirming their benign nature and origin.
Conclusion
XGC is till date an entity which can mimic malignancy pre operatively. Also, the reporting pathologists must be aware of the histology of developmental variants with ducts of Luschka, as confusion with other neoplastic and non-neoplastic conditions can occur specially, in cases of florid proliferation accompanied by marked inflammatory atypia, where biliary duct markers can be used for confirmation.
[1]. Barcia JJ, Histologic analysis of chronic inflammatory patterns in the gallbladder: diagnostic criteria for reporting cholecystitisAnn Diagn Pathol 2003 7(3):147-53. [Google Scholar]
[2]. Rammohan A, Cherukuri DS, Sathyanesan J, Palaniappan R, Govindan M, Xanthogranulomatous cholecystitis masquerading as gallbladder cancer: can it be diagnosed preoperatively?Gastroenterology Research and Practice 2014 2014:253645 [Google Scholar]
[3]. Rastogi A, Singh DK, Sakhuja P, Gondal R, Florid xanthogranulomatous cholecystitis masquerading as invasive gallbladder cancer leading to extensive surgical resectionIndian J Pathol Microbiol 2010 53:144-47. [Google Scholar]
[4]. Zhang LF, Hou CS, Liu JY, Xiu DR, Xu Z, Wang LX, Strategies for diagnosis of xanthogranulomatous cholecystitis masquerading as gallbladder cancerChin Med J (Engl) 2012 125(1):109-13. [Google Scholar]
[5]. Singh VP, Rajesh S, Bihari C, Desai SN, Pargewar SS, Arora A, Xanthogranulomatous cholecystitis: What every radiologist should knowWorld Journal of Radiology 2016 8(2):183-91. [Google Scholar]
[6]. Goshima S, Chang S, Wang JH, Kanematsu M, Bae KT, Federle MP, Xanthogranulomatous cholecystitis: diagnostic performance of CT to differentiate from gallbladder cancerEur J Radiol 2010 74(3):e79-83. [Google Scholar]
[7]. Kai K, Organ-specific concept and controversy for premalignant lesions and carcinogenesis of gallbladder cancerHepatobiliary Surgery and Nutrition 2016 5(1):85-87. [Google Scholar]
[8]. Hong YU, Tu-nan YU, Xiu-jun CAI, Tumor biomarkers: help or mislead in the diagnosis of xanthogranulomatous cholecystitis?—analysis of serum CA 19-9, carcinoembryonic antigen, and CA 12-5Chin Med J 2013 126:3044-47. [Google Scholar]
[9]. Rajab R, Meara N, Chang F, Florid ducts of Luschka mimicking a well differentiated adenocarcinoma of the gallbladderThe Int J Pathology 2007 6:360-65. [Google Scholar]
[10]. Singhi AD, Adsay NV, Swierczynski SL, Torbenson M, Anders RA, Hruban RH, Hyperplastic luschka ducts: a mimic of adenocarcinoma in the gallbladder fossaAm J Surg Pathol 2011 35(6):883-90. [Google Scholar]
[11]. Ewelukwa O, Ali O, Akram S, Xanthogranulomatous cholecystitis mimicking gallbladder cancerBMJ Case Rep 2014 2014:bcr2013200530 [Google Scholar]