Oral mucositis has severe physical and mental disability during the course of the treatment prompting interventions either to prevent such occurrence or treat them. The prevalence of oral mucositis is usually gravitated on type of cancer and its therapy. Generally patients treated with radiotherapy have the oral mucositis prevalence of about 100 % when compared to those in chemotherapy treated ones which is about 40% [1]. Oral mucosal erythaema appears in second week of radiotherapy with standard hyper fractionated doses of 2 Gy schedule. Administration of parenteral nutrition due to dysphagia and ulceration is a common presentation in 3rd-4th week of treatment. Nevertheless healing occurs by 5th-6th week of therapy with complete resolution. The side effects of radiotherapy include direct cell damage (epithelial) and production of reactive free radicals. Whereas in patients treated with chemotherapy the cell mitosis is affected during cell division [1-4].
Increased oral mucositis was commonly observed with bolus and continuous infusions when compared to repetitive lower dose chemotherapy. It occurs uniformly but was reported with reduced intensity in anterior oral mucosa and of short duration than that seen in radiotherapy patients [4-6].
Vitamin supplementation in the form of tablets, capsules or pastes have been tried with varying degree of success in both chemo/radiotherapy induced oral mucositis [1,3,4,7]. Vitamins like vitamin A, vitamin E and their combinations have been tried on oral mucositis. Vitamin A has an inhibitory effect on inflammation and epithelial proliferation. Vitamin E was found to be exerting antioxidant properties as well as scavenging free radicals released during inflammation. The efficacy of various other vitamins on its prevention or treatment is sparse in literature. A systematic review or systematic review with Meta-analysis is warranted to highlight such findings available as evidence.
The objective behind carrying out this analysis was to evaluate the efficacy of vitamins individually used for prevention or treatment of oral mucositis separately for chemotherapy, concurrent chemo radiotherapy, and radiotherapy and HSCT individuals.
Materials and Methods
The literature study was carried out using the references from medical data bases such as PUBMED, MEDLINE, EBSCO, GOOGLE SCHOLAR and COCHRANE data bases with search terms vitamin A, vitamin E, Oral mucositis, Chemotherapy, Radiotherapy, Concurrent chemo radiotherapy and HSCT individually.
Research Methodology
Inclusion and Exclusion criteria: Invitro studies, cell culture studies, studies on animals, research which were in combination with other dietary multivitamins, herbal medications, clinical reviews, case reports, research from other languages than English, abstracts only were discarded from study analysis. Only studies pertaining to individual vitamins on oral mucositis during cancer chemo-radiotherapy were considered. Combination of vitamin supplements was also considered. Additionally research studies on vitamin supplementation in the form of tablets, capsules or pastes were included in the analysis. Articles with full accessibility were included in the study. Those studies which had outcome measures of quality of life were also discarded as they did not fit in the aims and objectives of the study.
Data Retrieval: The study search included articles from year 1980- till May 2016. Only Randomized Controlled Trials (RCTs) were included in the search. In case of paucity of RCTs only then case control and cohort studies were used. The quality of reviewed literature was assessed by Hadorn’s DC et al., criteria [8].
The study was carried out by six research associates in the Department of Oral Medicine, Saveetha University, Chennai, Tamil Nadu, India, who were given designations as principal investigators, co investigators, and coordinators in the study.
An overview search of articles in the medical databases was initially screened by coordinators individually. They were supervised by coinvestigators who were available to clarify and evaluate articles in case of discrepancies with in the search by coordinators. The article retrieved by coordinators was approved by coinvestigators which were again approved by principal investigator. Any discrepancies within any source of article, incomplete references were discarded by the principal investigator who stood as final authority for the approval of searched items in the study. Coordinator 1 was allotted the task of searching radiotherapy induced oral mucositis and vitamin treatment related article, Coordinator 2 was allotted the task of evaluating the oral mucositis caused by concurrent chemo radio therapy related articles and Coordinator 3 was allotted to chemo therapy induced oral mucositis articles.
All the articles were divided into three separate groups (Group 1- radiotherapy, Group 2- concurrent chemo radiotherapy, Group 3- chemotherapy) were analysed and data was extracted utilizing the eligibility, validity and design of the study. The data was tabulated into excel format which included number of patients, grading of mucositis, type of treatment, type of vitamin supplementation, outcome measures, gender and age distribution in each for all three groups separately.
The output was assessed using binary random effects model. The primary analysis was designed to obtain or identify the grading of oral mucositis with the type of vitamin supplementation, their usage form and any effects of vitamins on oral mucositis separately for each group; CI intervals of 95% was considered for oral mucositis reduction as outcome; p-value at 0.05 was attributed as significant.
Levels of Evidence: Level I studies: Only RCTs were considered for the analysis taking the criteria of age and gender distribution, WHO grading of oral mucositis and intervention limited to only vitamin use for oral mucositis without any other primary objective.
Level II (a) studies were considered in cases of paucity of Level I studies. Level II (b) and Level III were excluded from the study.
Results
Number of articles totally reviewed was 40,203 all together in three groups. Number of articles which could not be retrieved belonged to concurrent chemo radio therapy as they did not fit into the objective of the study. The RCTs retrieved were 1329. Out of which the animal studies were 795 in number, which were excluded. Out of 534 articles, only 425 articles were considered. 109 articles were not considered as they were not accessible, only abstracts were retrieved and were in a language other than English. Chemotherapy related articles having vitamin E only were seven. Radiotherapy related articles having only vitamin E were just one. HCST related articles were two in number. Only one article had vitamin A as a formulation. No articles of chemotherapy along with vitamin A or radiotherapy along with vitamin A were considered due to: poor randomisation, poor grading pattern of oral mucositis or in combination with many other dietary micronutrients and minerals. The entire data has been tabulated in according to PRISMA flow chart [Table/Fig-1].
PRISMA flow chart on the number of manuscripts retrieved.
CT: Chemotherapy, RT: Radiotherapy, QoL: Quality of life
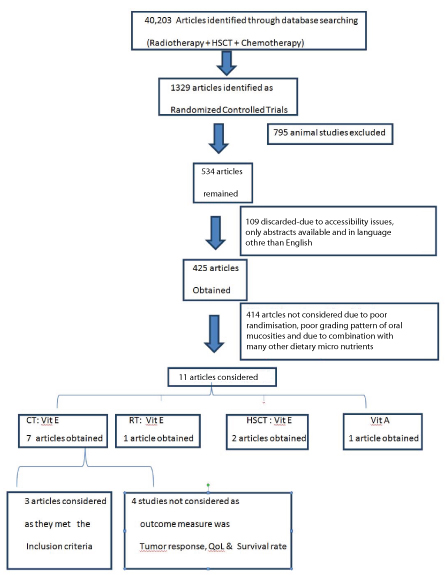
Chemotherapy related search result: Total number of studies from literature retrieved were seven which used vitamin E orally in cancer patients undergoing chemotherapy but only three studies complied with our objective and four others were excluded as the outcome measures were not measurement of oral mucositis but was tumour response, quality of life, and survival rate. The three studies which included were, Wadleigh RG et al., Azza A EI et al., and Arash AS et al., [9-11].
Many studies which focussed on combination of Vitamin E, Vitamin C, and others were not considered due to the different outcome measures related to these studies [12].
A study by Meyskens FL et al., was the only study that concluded that vitamin A oral supplementation (50,000 IU/day) on 124 patients caused increased oral toxicities of about 23% when compared to 4% in control group. The study did not precisely fit in our objective, though there was a reference on oral toxicity response in the study population [13].
Radiotherapy related search result: An exhaustive search was carried out on oral mucositis induced by radiotherapy in cancer patients and various supplementation used as a preventive or therapeutic intervention for treating the same. Out of articles searched, only one could fit our objective and was included in the analysis. Ferreira PR et al., studied Vitamin E in topical form and graded the response of supplementation on oral mucositis with a placebo control [14].
Concurrent chemo-radiotherapy and haematopoietic stem cell transplantation related search result: Three studies had fit into the criteria of search. Studies by Ghoreshi Z et al., Thornley I et al., Cohen G et al., had reported outcome objectives on assessing the severity of oral mucositis using vitamins. Vitamin A was studied by Cohen G et al., and vitamin E by Thornley I et al., and Ghoreshi Z et al. Additionally the study by Thornley I et al., had a combination treatment of systemic supplementation of Vitamin E and another drug UCDA for oral mucositis in HSCT individuals. It is to be noted that there were no RCTs in the literature search which focussed on the aspect of oral mucositis involving vitamin supplementation using concurrent chemo radio therapy protocols [15-17].
Grading of oral mucositis: The grading of oral mucositis during the cancer treatment plays a vital role in assessing the response of the patient towards the treatment. Several scoring systems have been devised to grade the severity of oral mucositis and its sequelae. Nevertheless uniformity in usage of such scales was achieved. Two most commonly used scales were WHO and NCI-CTC (National Cancer Institute Common Toxicity Criteria). Apart from these, customised scales also were used in some studies but validity and reproducibility of the same, needs evaluation at a large scale.
The studies retrieved and used in this analysis used WHO grading of oral mucositis and NCI-CTC scales. Under the chemotherapy group, Wadleigh RG et al., Azza A El et al., Arash AS et al., had used WHO grading scales [9-11]. In radiotherapy group Ferreira PR et al., used RTOG/EORTC criteria of oral mucositis grading [14]. In HSCT group, two studies, Ghoreshi Z et al., and Thornley I et al., had used NCI-CTC grading [15,16], where as Cohen G et al., used Seto BG et al., grading of mucositis [17,18].
Vitamins prescribed under topical formulations: Only three studies i.e., Azza A El, Wadleigh RG et al., and Ferriera PR et al., were considered for analysis as considerable heterogeneity was observed for an analysis involving topical and systemic medications in many other studies. No Meta-analysis was performed for systemic medication group with heterogeneous data. Weights of samples considered for analysis were 9.4% for Wadleigh RG et al., study, 37.45% for Azza A EI et al., study and 53.1% for Ferriera PR et al., study [9,10,14].
Binary random effects model for metric proportion was performed on the studies with standard error of 0.049 and keeping p-value significance at <0.001. The association between Vitamin E topical usage in oral mucositis with clinical reduction of the same was estimated with odd’s ratio and relative risk at 95% confidence interval. Out of 67 patients treated with topical vitamin E, 65 patients had significant reduction of oral mucositis during cancer therapy. Odd’s ratio obtained was 11.2 which demonstrated a good outcome in this regard [Table/Fig-2].
Meta analysis of the topical vitamin medication in oral mucositis.
 |
| Topical application:Weightages:Study names weightsAZZA EL HOUSSEINY 2007: 37.458%ROBERT G 19992 : 9.415%FERREIRA 2004 : 53.127%Binary Random-Effects ModelMetric: ProportionModel Results |
| Estimate | Lower bound | Upper bound | Std. error | p-value |
| 0.837 | 0.740 | 0.933 | 0.049 | < 0.001 |
| Heterogeneity |
| tau^2 | Q(df=2) | Het. p-Value | I^2 |
| 0.001 | 2.333 | 0.311 | 14.267 |
Discussion
The main complication of non surgical treatment of cancer is oral mucositis. It is considered an inherent outcome of chemotherapy or radiotherapy to head and neck region in cancer patients. Public health prospective on oral mucositis is a larger issue having significant role in physical and psychological aspects of cancer patients [1,2].
In addition to prescriptions of such agents for oral mucositis, it is a common practise among many oncologists to prescribe multivitamin supplements during the entire treatment process. Dietary micro nutrients find their way into the diet chart of the patients during chemo or radiotherapy [2,6,7,9].
Reports of the quality of evidence and outlier data: The value of significant outcome in the reduction of oral mucositis among the samples searched with systemic application of Vitamin A and Vitamin E individually were included for systematic analysis
1) Gender Distribution - Total number of males in case groups were 68;
Total number of males in control groups were 56 (n=56);
Total number of females in case groups were 46;
Total number of females in control groups were 31 (n=31).
The analysis clearly demonstrated that in both cases and control groups there appeared a male predominance to females
2) Age Distribution- Out of eight studies, two had evaluated paediatric population with the mean ages of 5.75±3.38 having topical Vitamin E application and 9.3±2.44 using systemic Vitamin E administration (Azza A EI et al.,) [10]. The study by Thornley I et al., had mean age of eight years in both study and control groups [16]. Remaining six studies concentrated on adult population.
3) Grading of Oral Mucositis: It was done using WHO, NCI-CTC criteria and customized Seto BG et al., criteria [18]. Presence of heterogeneity led to only consideration for systematic analysis.
Inference From Results
Analysis of studies on Vitamin E Systemic Administration in all Three Groups: The analysis revealed that four studies have used Vitamin E systemic administration in varying doses. Some have compared their topical usage with controls. All had used twice daily medications. Two studies among four had used Vitamin E oral supplementation before the initiation of cancer treatment. The studies reported variable reduction of oral mucositis using Vitamin E pills. Topical Vitamin E group in all three cancer treatment modalities showed significant reduction in oral mucositis when compared to systemic group. This study by Thornley I et al., had used combination of Vitamin E and Ursodeoxycholic acid (USDA), Folenic acid and parenteral nutrition [16]. The study demonstrated better outcome in case group when compared to placebo [Table/Fig-3].
Details of studies included in the review and dosages of vitamins used in treatment of oral mucositis during cancer treatment.
| Type of Treatment | Study | Vitamin | Sex | Age | Number Of Patients | Topical | Systemic | Grading Scale |
|---|
| Chemo-therapy | Wadleigh RG et al., [9] | E | Not available | 53-67 yrs | 18 | 1 ml of vit E oil, 400 mg/ml, twice a day for five days | _ | WHO |
| Azza A EI et al., [10] | E | Not available | < 12 yrs | 63 | 100 IU capsule to be emptied into oral cavity twice daily and followed for five days | 100 IU soft gel capsule to be swallowedtwice daily and followed for five days | WHO |
| Arash AS et al., [11] | E | Male: 41Female: 35 | ≥ 18 yrs | 76 | I gm of vit E paste to be applied twice daily, two days before the treatment till at least 20 days after completion of each cycle | 200 mg of pills to be taken twice daily, two days before the treatment till at least 20 days after completion of cycle | WHO |
| Radiot-herapy | Ferreira PR et al., [14] | E | Male: 48Female:6 | Mean age 53.5 yrs | 54 | _ | 400 mg vit E for five days twice daily. Rinse for five minutes and then swallow. | EORTC/ RTOG |
| HSCT | Ghoreshi Z et al., [15] | E | Male: 28Female :32 | ≥10 yrs, Mean age 27 yrs | 60 | _ | 400 mg twice daily at seven days before BMT until 28 days after BMT | NCI-CTC |
| Thornley I et al., [16] | E | Not available | Mean age 8 yrs | 168 | _ | 8 IU/Kg once daily if body weight<25 kg400 IU/Kg once daily if body weight>25 kg. 3-5 days before conditioning and continued through day 27 after transplantation or discharge from HSCT unit, whichever occurred first. | NCI-CTC |
| Cohen G et al., [17] | A | Male: 7Female: 4 | Mean age 34.8±9.8 yrs | 11 | Twice daily 0.25 mg of 0.1% tretinoin ointment. Duration was from the beginning of BMT conditioning regimen until day +21 post BMT. | _ | SETO et al. |
Analysis of Studies on Vitamin A in all Three Groups: Out of eight studies, only one study in HSCT group studied Vitamin A ointment (0.1%) once daily application of 0.25 mg Tretinion. The study reported reduced oral mucositis in a small group of patients (p-value significant) when compared to controls [Table/Fig-3].
Limitation
Paucity of literature using various other vitamins such as Vitamin C or Vitamin B individually or in combination had been one of the limitations. A well controlled Randomized Trial with larger sample, uniform grading scale seemed essential for reduction of this invariable side effect which might alleviate symptoms of these patients improving their quality of life.
Conclusion
The present analysis demonstrated that Vitamin E topical application had performed better on oral mucositis than Vitamin E systemic administration. The efficacy of Vitamin A topical treatment also showed reduction in severity of oral mucositis similar to vitamin E. It has to be noted that the analysis was carried out on the sample size with small number of retrievable articles in the literature. Presence of considerable heterogeneity in assessing the treatment of oral mucositis, outcome measures and difference in treatment application led to inconclusive evidence so as to highlight which formulation had best possible outcome in reducing oral mucositis.