A 60-year-old postmenopausal woman, presented with a left ovarian cystic mass with mildly elevated CA125 levels. An intraoperative frozen section showed oedematous ovarian stroma with interspersed large aggregates of spindle shaped stromal cells. Subsequently, the excised specimen was reported as Sertoli-Leydig Cell Tumour (SLCT) of intermediate differentiation. The leydig cells were identified in the imprint smears, but were misinterpreted as luteinized cells. The lack of tubular differentiated cells in frozen section had contributed to the misdiagnosis. Immunohistochemistry (IHC) played an important diagnostic role in the absence of clinical suspicion and lack of virilising features that are classically described in association with SLCTs.
This case is unusual, as the tumour was seen in a postmenopausal woman in the absence of virilising symptoms. The cytomorphological features, IHC findings and the reasons for misdiagnosis are discussed in this case report.
Case Report
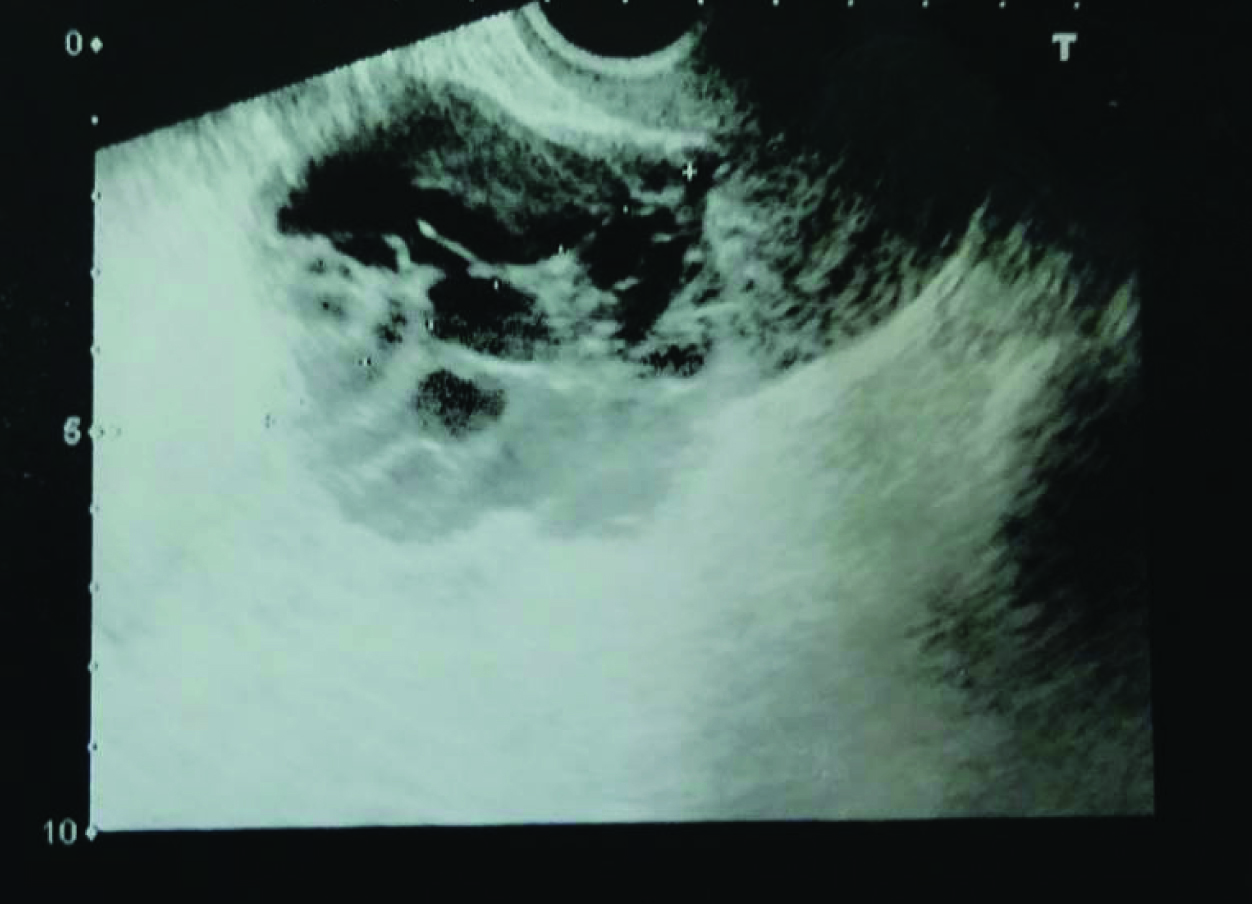
A 60-year-old postmenopausal woman, known diabetic and hypertensive presented with spasmodic pain of one month duration in left lower abdomen. On clinical examination, abdomen was soft, non-tender with adiposity of abdominal wall. Per-speculum examination – cervix and vagina were healthy. On per-vaginum examination uterus was normal size, mobile, retroverted and b/l fornices were free and non-tender. Our patient did not show endocrine features. Trans-vaginal ultrasonographic examination revealed a complex cystic mass involving left ovary, with multiple internal septations measuring 8 x 5 cm [Table/Fig-1]. There was no free fluid in the abdomen or pelvis. CA125 was 22 IU/ml.
USG image showing cystic lesion in left adnexa with multiple thick and thin internal septations.
Portion of the cyst was sent for intraoperative frozen section.
Intraoperative Imprint and Frozen Section Findings
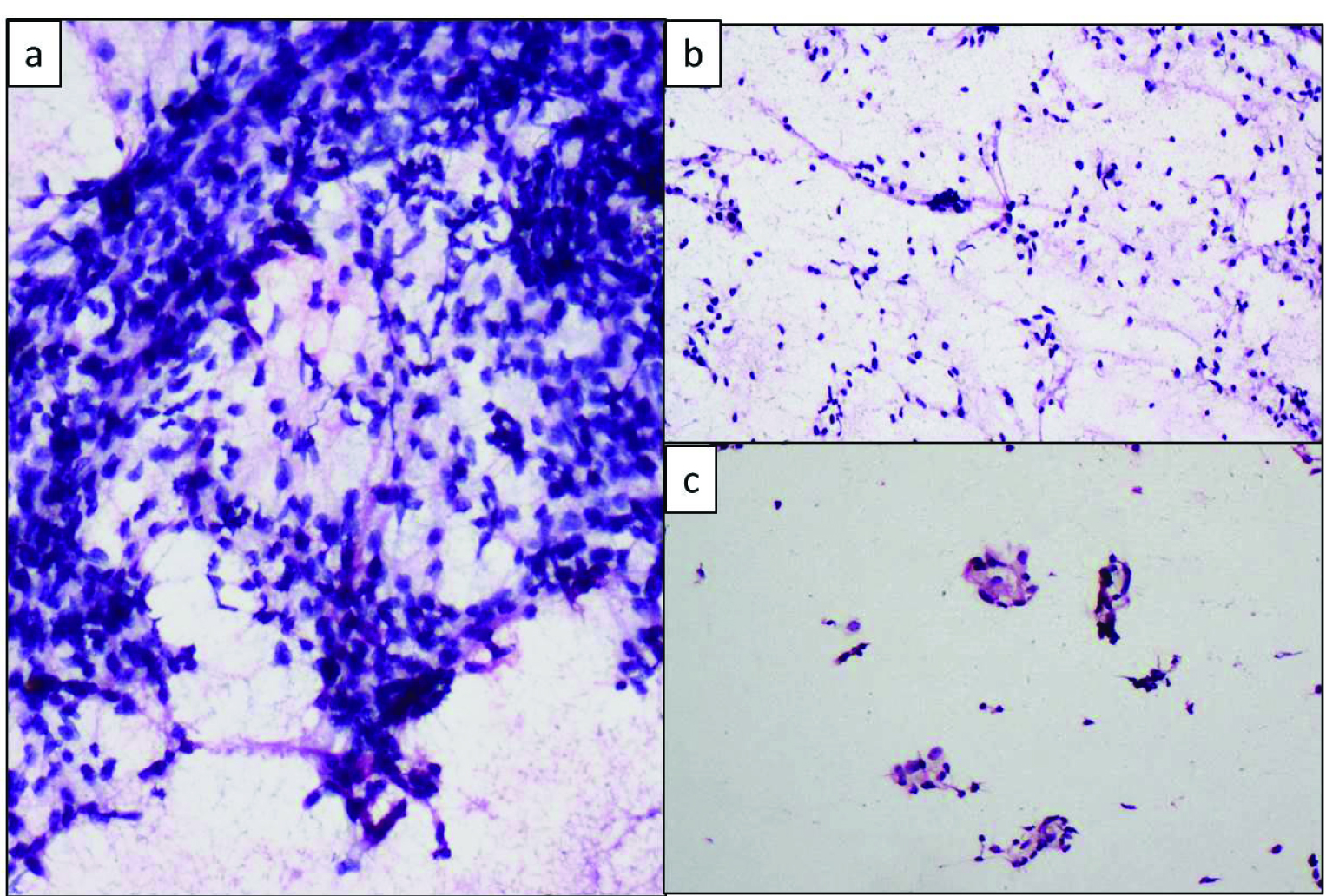
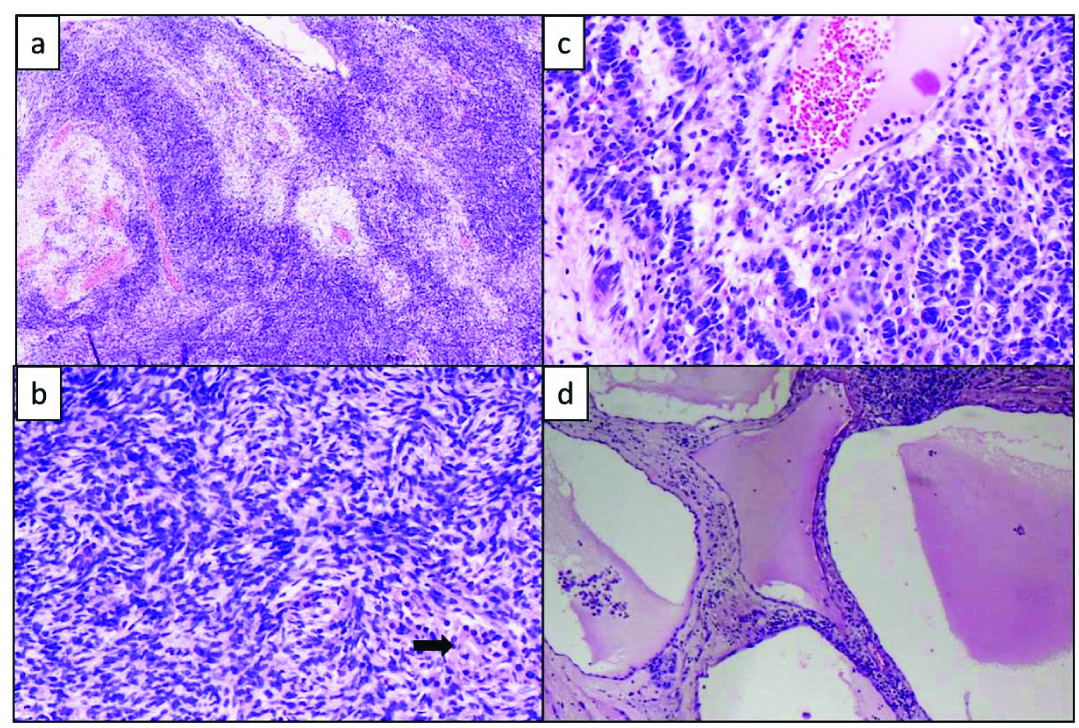
Intraoperative imprint cytology of the frozen section bit showed large aggregates of plump spindle shaped cells in a background of bare nuclei and occasional clusters of cells with abundant eosinophilic cytoplasm (Leydig cells) which were misinterpreted as luteinized cells [Table/Fig-2].
Imprint smears showing: a) Clusters of plump spindle cells (Rapid H&E, 40X); b) Bare nuclei, (Rapid H&E, 20X); c) Leydig cell clusters which were misinterpreted as leutinised cells (Rapid H&E, 20X).
Frozen section showed oedematous ovarian stroma with interspersed large aggregates of spindle shaped cells [Table/Fig-3a,b]. The frozen section in conjunction with imprint cytology was reported as oedematous ovarian stroma. No evidence of malignancy was noted.
Frozen section showing: a) Oedematous ovarian stroma (H&E, 20X); b) Higher power view of the stromal cell clusters (H&E, 40X); c) Frozen bit, processed for routine histopathology showing leydig cells at the periphery of the large stromal cell clusters, H&E, 40X) (left to right).
In view of the clinical suspicion of malignancy, a completion surgery of total abdominal hysterectomy with right salphingo- oophorectomy amd left ovariotomy with salphingectomy was performed.
Histopathological Findings
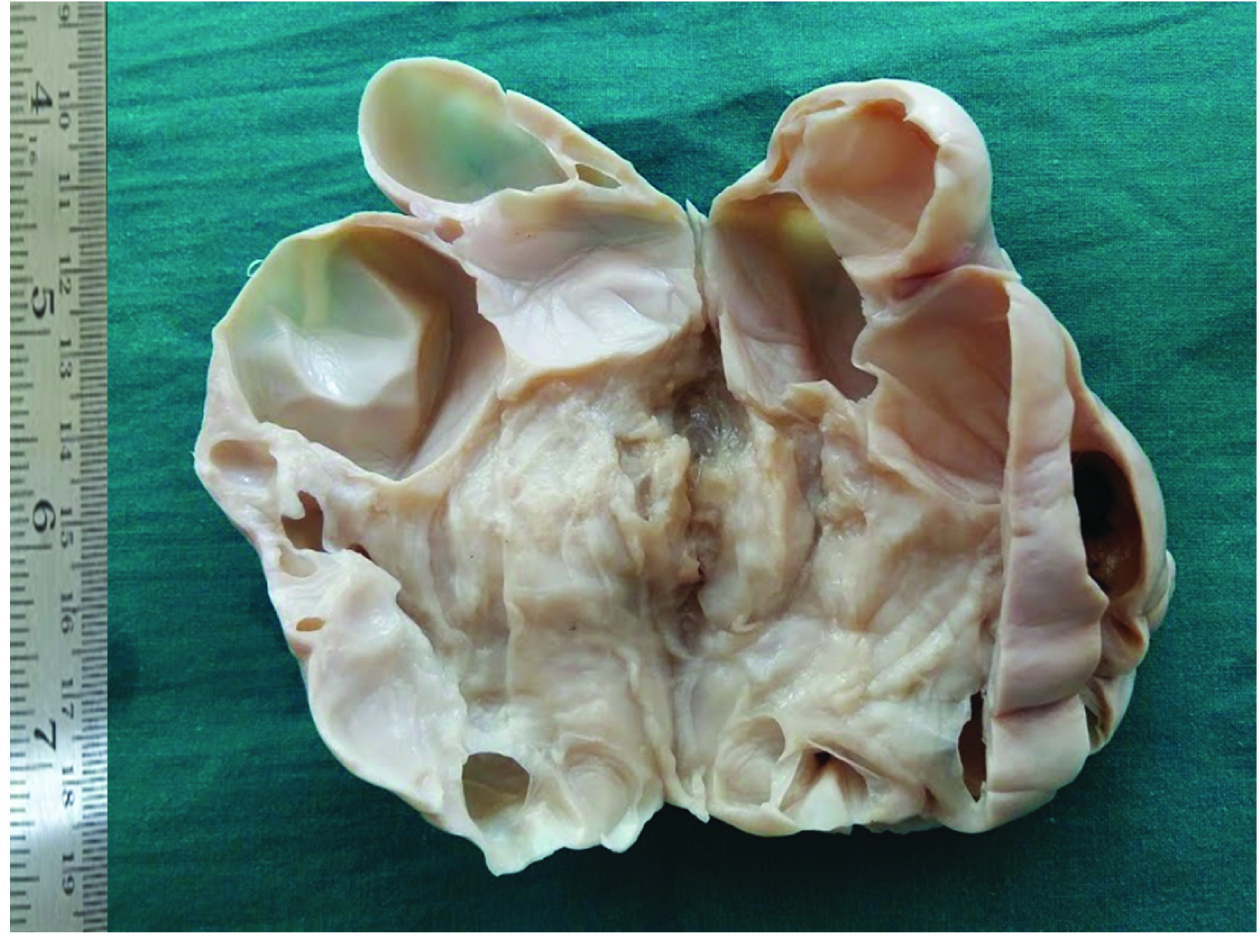
Grossly, the uterus was atrophic. Separately sent left ovary measured 8.5x5x4 cms, was predominantly cystic, multiloculated with thick septations and luminal clear fluid [Table/Fig-4]. Right ovary and tube were not involved.
Gross image showing predominantly cystic left ovary with thick internal septations focal solid areas.
The frozen section bit was processed for routine histopathology and showed variably oedematous stroma with scattered irregular nodules of haphazardly arranged plump spindle shaped cells, stromal cells with interspersed ill-defined nests of cells with enlarged oval nucleus, vesicular chromatin, inconspicuous nucleolus and amphophilic cytoplasm (immature sertoli component). Scattered small groups of cells with granular eosinophilic cytoplasm and enlarged nucleus (leydig cells) were seen [Table/Fig-3c].
Sections from left ovarian mass showed a cystic-solid tumour composed of scattered nodules of low columnar cells with high N:C ratio with large pleomorphic, hyperchromatic nuclei, coarse chromatin, with few cells showing prominent basophilic nucleoli with increased mitosis (5/10 HPF) arranged in anastomosing cords and nests (mature sertoli component). Focal glandular differentiation in the form of tubular glands noted. Peripheral portion showed nests of large cells with abundant eosinophilic cytoplasm and central large nuclei (leydig cells) [Table/Fig-5]. No heterologous elements were noted.
Routine Histopathology: a) Section shows large spindle cell clusters separated by oedematous stroma (H&E,10X); b) Spindle cells clusters with interspersed leydig cells with abundant eosinophilic cytoplasm (H&E, 20X); c) More differentiated Sertoli cells arranged in cords with interspersed leydig cells (H&E, 20X); d) Other areas showing the predominantly cystic component (H&E, 10X).
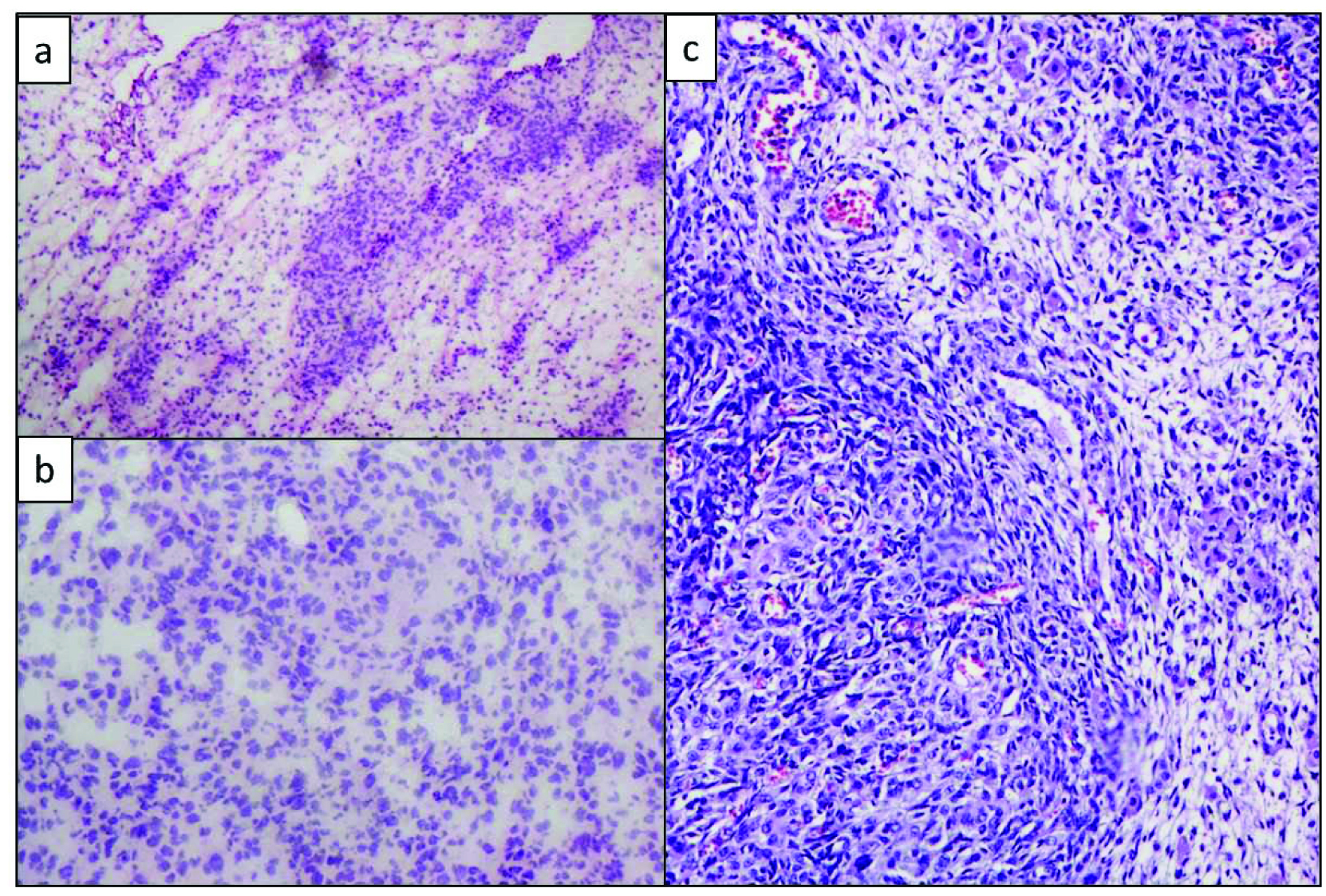
Inhibin was diffusely positive in both the sertoli and leydig cell components [Table/Fig-6a]. Cytokeratin (CK) was positive in the mature and immature sertoli cell component [Table/Fig-6b]. Vimentin was positive in the leydig cells [Table/Fig-6c].
a) Diffuse inhibin positive in both sertoli and leydig cell components, Inhibin, (IHC, 40X); b) CK positivity in sertoli cell component, CK (IHC, 20X); c) Vimentin positivity highlighting the leydig cells, Vimentin (IHC, 40X)
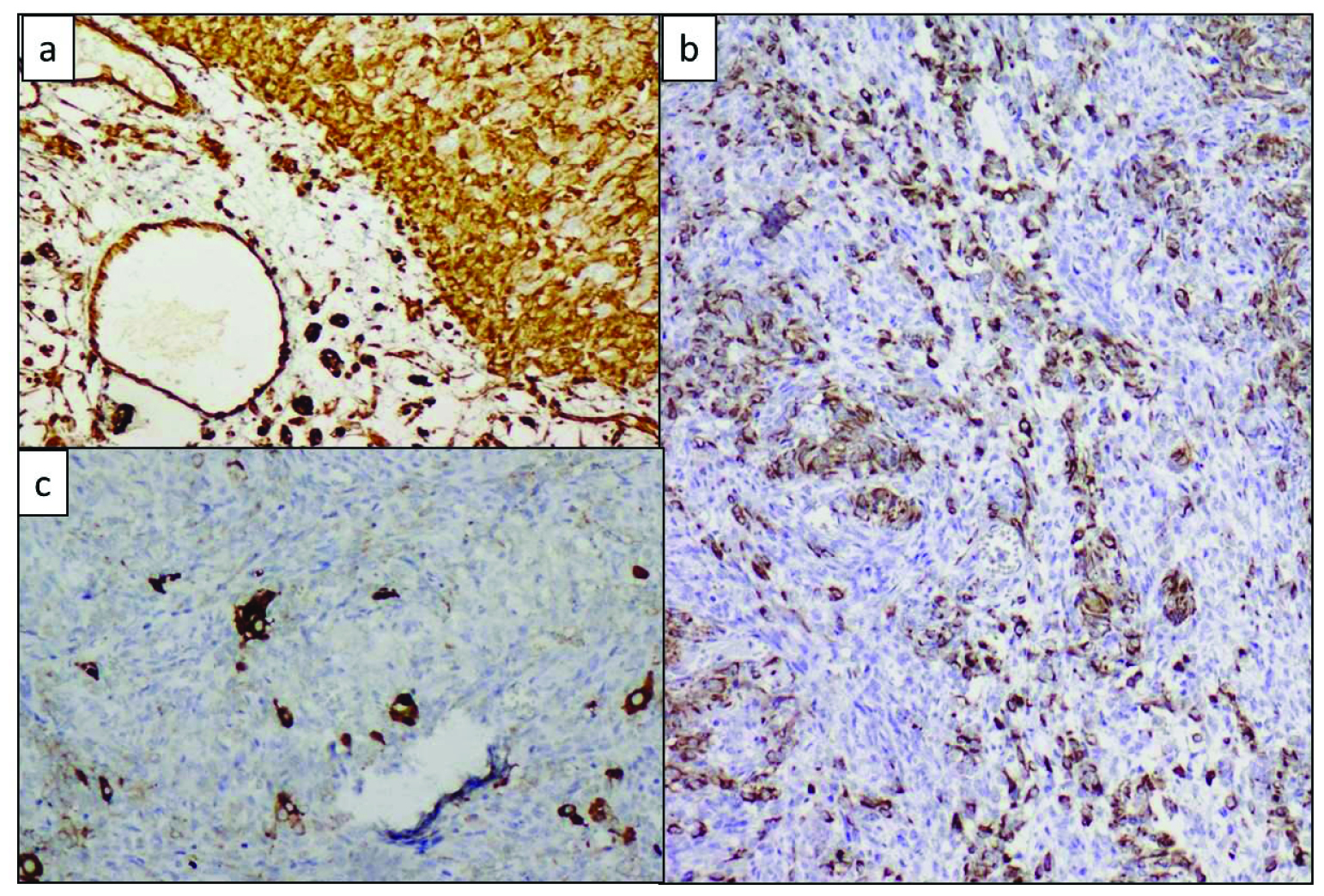
A final diagnosis of SLCT, intermediate differentiation was made based on the histomorphology and IHC findings.
Post-surgical CT scan of thorax, abdomen and pelvis did not show evidence of metastatic tumour deposits. Patient was on regular follow up for a period of one year with no history of tumour recurrence or metastasis.
Discussion
SLCT are rare tumours accounting for less than 0.5% of all ovarian neoplasms [1]. They are composed of a mixture of variable proportions of cells morphologically resembling male sertoli and leydig cells. The WHO classifies them under sertoli-stromal cell tumours.
SLCT commonly occurs in young women and is rare after menopause [2]. Signs of androgen excess are seen in around 50% of the cases. This is manifested as defeminization (amenorrhea, breast atrophy, loss of sub-cutaneous fat) followed by masculinization (hirsutism, deepening of voice, clitoral hypertrophy). There is prompt return of feminine characteristics with excision of tumour; however the disappearance of masculinization features is slow [3]. Other lesions associated with androgen excess are polycystic ovarian disease, ovarian stromal hyperthecosis. Some patients present with estrogenic manifestations like menorrhagia/metrorrhagia or postmenopausal bleeding. Half of the cases have non-specific clinical features [4]. Our patient did not show endocrine features. Around 20% patients have elevated α Fetoprotein (AFP) derived from the heterologous hepatocyte component.
SLCTs are commonly unilateral and usually confined to the ovary at the time of diagnosis. Bilateral involvement is seen in less than 2% of the cases. Grossly, they are predominantly solid but cystic areas may be seen [5]. Our case was a multiloculated cyst with thick septations. The benign cystic appearance again contributed to the diagnostic confusion. Well-differentiated SLCTs are smaller in size with a mean diameter of 5 cm while intermediate and poorly differentiated tumours have an average diameter of 15 cm [6].
These tumours can occur in a variety of clinical settings: with benign and rarely malignant ovarian tumours, cervical sarcoma botryoides, pregnancy and thyroid diseases [2-7]. Androgen excess can be seen in non-neoplastic conditions like polycystic ovarian disease and ovarian stromal hyperthecosis which is associated with bilateral ovarian involvement. Hilus cell tumour, luteoma, gynandroblastoma, luteinized thecoma, gonadoblastoma, granulosa cell tumour and metastasis to the ovary can also show elevated androgen levels. Unlike virilising adrenal gland tumours, SLCTs do not show marked elevation of urinary 17-ketosteroids [7].
Ultrasound is regarded as a useful imaging technique for the initial assessment of SLCTs. Other imaging techniques like CT, MRI, and PET scans are more useful in tumour staging [4]. The present case was initially detected during transvaginal USG.
The histologic appearance of these tumours is extremely variable and has been categorized into the following groups [8,9]: (1) Well differentiated- solid or hollow tubules composed of sertoli cells surrounded by a fibrous stroma containing clusters of leydig cells. Lacks significant atypia or mitotic activity; (2) Intermediate differentiated – large cellular ‘blue nodules’ surrounded by an oedematous stroma. On careful high power scrutiny these nodules show cords and sheets of dark blue sertoli cells and eosinophilic leydig cells. Mitotic rate is around 5/10hpf; (3) Poorly differentiated (sarcomatoid) – dominant sarcomatoid stroma resembling primitive gonadal stroma. It lacks the lobular arrangement of intermediate differentiated tumours. Mitosis is high, around 20/10 hpf. A thorough search for cords of sertoli cells and leydig cells is essential for diagnosis; (4) With heterologous elements – mucinous epithelium of GIT, liver, endocrine cells, carcinoid, skeletal muscle, cartilage, rhabdomyosarcoma etc., (5) Retiform – 90% of the tumour is comprised of ramifying network of slit-like spaces resembling rete testis; (6) Pure Sertoli cell tumour – similar to well differentiated tumour but lacking the Leydig cells and stromal elements.
These tumours tend to show striking intercellular oedema [10] which can be overlooked as a benign finding. In the present case also frozen section was misdiagnosed as oedematous ovarian stroma as the immature sertoli component and leydig cells were not picked up. The authors wish to highlight this diagnostic pitfall. Presence of bizarre cells is a degenerative change and not a feature of malignancy.
IHC has proven diagnostic application. Sex cord elements show variable positivity for Vimentin, CK and α Inhibin while being negative for Epithelial Membrane Antigen (EMA). Leydig cell show intense positivity for Vimentin and α Inhibin and are negative for CK, CAM5.2 and AFP [11]. Similar findings were observed in the present case. EMA negativity helps to differentiate SLCTs from endometroid adenocarcinomas.
Molecular studies have not been done extensively for SLCTs. Trisomy 8 and trisomy 12 have been observed on cytogenetic analysis of these tumours [12,13].
Treatment options include surgery with or without adjuvant chemo and radiotherapy. In young women, where preservation of fertility is desired, unilateral salpingo-oophorectomy is done, if the tumour is stage IA. More aggressive surgical therapy with chemo/radiotherapy is indicated for advanced stage tumours and tumours with poor prognostic features [14,15]. Our patient had tumour confined to the ovary and was treated surgically with ovariotomy and hysterectomy and opposite side salphingo-oophorectomy. Now three years since surgery, the patient has not come back with tumour recurrence.
Prognosis of SLCTs is generally good and depends on the stage and degree of differentiation of tumours. Poor differentiation, presence of heterologous elements, high mitotic counts and rupture of tumour are associated with increased risk of metastasis [16].
Conclusion
To conclude, SLCTs are rare ovarian tumours belonging to the group of sex cord stromal tumours. Absence of endocrine manifestations, presentation in postmenopausal age group and grossly cystic mass with focal solid areas were the unusual features observed in this case. SLCTs has varied histomorphology and may show areas of oedema which may contribute to misdiagnosis particularly during frozen section. However, the presence of large sheets of spindle cells and randomly distributed leydig cells should prompt the pathologist regarding the possible diagnosis of SLCT.
[1]. Roth LM, Anderson MC, Govan ADT, Langley FA, Gowing NFC, Woodcock AS, Sertoli-Leydig cell tumour. A clinicopathologic study of 34 casesCancer 1981 48:187-97. [Google Scholar]
[2]. Young RH, Dudley AG, Scully RE, Granulosa cell, Sertoli-Leydig cell and unclassified sexcord-stromal tumours associated with pregnancy. A clinicopathological analysis of thirty-six casesGynecol Oncol 1984 18:181-205.2a – hindawi [Google Scholar]
[3]. Stegner H-E, Lisboa BP, Steroid metabolism in an androblastoma (Sertoli-Leydig cell tumour). A histopathological and biochemical studyInt J Gynecol Pathol 1984 2:410-25. [Google Scholar]
[4]. Zhang HY, Zhu JE, Huang W, Zhu J, Clinicopathologic features of ovarian Sertoli-Leydig cell tumoursInt J Clin Pathol 2014 7(10):6956-64. [Google Scholar]
[5]. Seidman JD, Patterson JA, Bitterman P, Sertoli-Leydig cell tumour associated with a mature cystic teratoma in a single ovaryModern Pathol 1989 2:687-92. [Google Scholar]
[6]. Zaloudek CF, Tumours of the female genital tract. In: Fletcher DM, editorDiagnostic Histopathology of Tumours 2007 3rd edLondonChurchill Livingstone Elsevier:595-98. [Google Scholar]
[7]. Zanotti KM, The clinical manifestations and diagnosis of Sertoli-Leydig cell tumours of the ovaryJournal of Gynecologic Oncology 2002 7:129-33. [Google Scholar]
[8]. Roth LM, Recent advances in the pathology and classification of ovarian Sex cord –stromal tumoursInt J Gynecol Pathol 2006 25:199-215. [Google Scholar]
[9]. Young RH, Sex cord-stromal tumours of the ovary and testis: their similarities and differences with consideration of selected problemsModern Pathology 2005 18:S81-S98. [Google Scholar]
[10]. Young RH, Sertoli-Leydig cell tumours of the ovary. Review with emphasis on historical aspects and unusual variantsInt J Gynecol Pathol 1993 12:141-47. [Google Scholar]
[11]. Tavassoli FA, Devilee P, World Health Organization Classification of tumoursPathology and genetics of tumours of the breast and female genital organs 2003 LyonIARC press:146-61. [Google Scholar]
[12]. Manegold E, Tietze L, Gunther K, Fleischer A, Amo-Takyi BK, Schröder W, Trisomy 8 as sole karyotypic aberration in an ovarian metastasizing Sertoli-Leydig cell tumourHum Pathol 2001 32:559-62. [Google Scholar]
[13]. Taruscio D, Carcangiu ML, Ward DC, Detection of trisomy 12 on ovarian sex cord stromal tumours by fluorescence in situ HybridizationDiagn Mol Pathol 1993 2:94-98. [Google Scholar]
[14]. Young RH, Scully RE, Ovarian Sertoli-Leydig cell tumour. A clinicopathological analysis of 207 casesAm J Surg Pathol 1985 9:543-69. [Google Scholar]
[15]. Liggins CA, Ma LT, Schlumbrecht MP, Sertoli-Leydig cell tumour of ovary: A diagnostic dilemmaGynaecologic Oncology Reports 2016 15:16-19. [Google Scholar]
[16]. Zaloudek C, Norris HJ, Sertoli-Leydig cell tumours of the ovary. A clinic-pathological study of 64 intermediate and poorly differentiated neoplasmsAm J Surg Pathol 1984 8:405-18. [Google Scholar]