Introduction
In the era of drug-eluting stents, Bare Metal Stent (BMS) has worked its way up to be recognized in several indications. Moreover, literature suggests that strut thickness has been directly related to the restenosis rate.
Aim
We intended to evaluate the clinical performance of the ultrathin (60 μm) Flexinnium stent (Sahajanand Medical Technologies Pvt. Ltd. Surat, India) for treatment of a wide range of patients with coronary artery disease in routine clinical practice.
Materials and Methods
This was an observational, non-randomized, retrospective, single-arm study carried out in real-world patients at three clinical centres of India. A total of 419 consecutive patients’ data was collected for the study, who underwent treatment for coronary lesions by implantation of Flexinnium stent, between April 2013 and December 2014. The primary endpoint of the study was Major Adverse Cardiac Events (MACE), a conglomerate of cardiac death, Myocardial Infarction (MI) (Q-wave and non-Q-wave), Target Lesion Revascularization (TLR) and Target Vessel Revascularization (TVR). Any incidence of Stent Thrombosis (ST) was also observed as safety endpoint. These endpoints were observed during in-hospital stay, at 30 days, six months and at 12 months follow up. All data were analysed using the Statistical Package for Social Sciences (SPSS; Chicago, IL, USA) program, version 15.
Results
A total of 491 lesions were treated in 419 patients having mean age of 54.1 years. A total of 525 Flexinnium stents were implanted. There were 243 (58.0%) patients with hypertension. At 12 months the total incidences of MACE were 14 (3.5%). These included 9 (2.3%) cardiac deaths, 1 (0.3%) MI, 3 (0.8%) TLRs and 1 (0.3%) TVR. There was one incidence of definite ST at 12 months follow up.
Conclusion
Our results demonstrate that the Flexinnium stent is associated with a low 12 months incidence of MACE in a wide range of real-world population. Long-term follow up would further confirm its clinical performance profile.
Introduction
Globally, cardiovascular diseases are the leading cause of death and serious illness accounting for 30% of the deaths annually [1]. Forty years ago, Coronary Artery Bypass Grafting (CABG) surgery was the popular revascularization treatment used in Coronary Artery Diseases (CAD) [2]. Since the dawn of Percutaneous Coronary Intervention (PCI) in 1980s, the number of PCIs performed each year has expanded considerably [1,3]. PCI was initially performed using balloons. Inflating a distensible balloon led to disruption and rearrangement of the plaque that otherwise led to ischaemia; however, in some patients, acute thrombosis, dissection of the artery, or elastic recoil after deflation of the balloon resulted in an inadequate lumen with subsequent symptomatic re-narrowing [4]. This resulted in decreased use of balloon angioplasty and was replaced by BMS.
As the years elapsed, the era budged from use of BMS to durable-polymer coated Drug-Eluting Stents (DES) to biodegradable-polymer coated DES to bioabsorbable stents. However, the BMS has still worked its way up to be recognized in several indications. Various clinical and economic situations of individuals contribute towards the use of BMS over DES. Recent guidelines endorse the use of balloon angioplasty or BMS in patients with high bleeding risk, inability to adhere to 12 months of Dual Antiplatelet Therapy (DAPT), or anticipated invasive procedure or surgery within 12 months, which would require DAPT interruption [5]. Elderly patients have a higher risk of bleeding as well as comorbidities, which upshots the preference of BMS over DES in such patients. Moreover, DESs have limited flexibility and deliverability [6], with variable penetration rates across the different areas, when compared to the BMSs [7]. Although, DESs have been associated with lower rates of restenosis when compared to BMS, but still pose a risk of late and very late stent thrombosis [8-10].
Literature suggests that the stents with thin struts have been associated with improved flexibility and deliverability, faster endothelialization and a lower rate of in-stent restenosis probably due to diminished vascular injury during implantation [11-13]. Flexinnium (Sahajanand Medical Technologies Pvt. Ltd., Surat, India) is one of the new generation BMS with ultrathin struts. The Flexinnium coronary stent system consists of L605 Co-Cr alloy located on balloon catheter in the midst of two radiopaque markers. Flexinnium stent, with ultrathin strut (60 µm), is developed with a highly flexible ‘S-link’ which allows the stent to be more flexible hence, more deliverable while it travels through vessels at the time of implantation. Thus, we intended to evaluate the clinical performance of the ultrathin (60 μm) Flexinnium stent for treatment of a wide range of unselected patients with CAD in routine clinical practice.
Materials and Methods
Study Design and Patient Population
This was an observational, non-randomized, retrospective, single-arm study carried out in real-world patients at three clinical centres of India. A total of 419 consecutive patients’ data was collected in the study, who underwent treatment for coronary lesions by implantation of Flexinnium stent, between April 2013 and December 2014. Before the discharge of the patient from the hospital, data release consent was taken from every patient, which is the practise of associated hospitals, irrespective of any study to be conducted in future. Baseline clinical data with procedural outcomes, patients were obtained from medical records and follow up data were recorded prospectively. The study was approved by the Institutional Review Board or Independent Ethics Committee.
The patients were included for analysis if: 1) patient’s age was 18 years or above; 2) patients had stable, unstable angina or acute recent myocardial infarction, and 3) were undergoing coronary intervention with the study stent. The patients were excluded if they declined to give consent for data release or if they had any allergy to aspirin, clopidogrel, ticlopidine, heparin and cobalt chromium.
Description of the Study Stent
The Flexinnium coronary stent system comprises L605 Co-Cr alloy positioned on balloon catheter amid two radiopaque markers. High tensile properties of Co-Cr alloy lead to thinner struts without compromising the radial strength. The Co-Cr alloy has been preferred as it retains the properties of biocompatibility, radiopacity, strength, non-ferromagnetism, and high corrosion resistance. Flexinnium has been developed with a very flexible ‘S-link’ that allows the stent to be more flexible hence, more deliverable as it travels through vessels during implantation. Strut is ultrathin with a thickness of 60 µm. Flexinnium stent is available in different lengths i.e., 8, 12, 16, 20, 24, 28, 32, 36, 40, 44 and 48 mm and different diameters i.e., 2.0, 2.25, 2.5, 2.75, 3.0, 3.5, 4.0, and 4.5 mm.
Interventional Procedure and Adjunctive Medications
All patients were given a loading dose of 300 mg of aspirin and clopidogrel (300 mg) or prasugrel (60 mg) or ticagrelor (two tablets each of 90 mg). The procedural anticoagulation was brought about either with heparin or bivalirudin. The intra-procedural glycoprotein IIb/IIIa- inhibitor was administered based on the investigator’s decision. The procedure was performed as per standard treatment guidelines of every participating centre. Dual antiplatelet therapy (aspirin 75-300 mg daily indefinitely and clopidogrel 75 mg daily or prasugrel 10 mg daily or ticagrelor 90 mg twice daily for at least 1 month) was administered to all patients after the procedure.
Endpoints of the Study
Occurrence of any MACE was the primary end-point of the study. The MACE was defined as a miscellany of cardiac death, MI (Q-wave and non-Q-wave), TLR and TVR. The occurrence of ST was also analysed as safety endpoint as per the Academic Research Consortium. These endpoints were observed at in-hospital stay, 30 days, six months and 12 months follow up. The secondary endpoints will be measured at 24 months.
Definitions of Endpoints and Clinical Events
Any death due to a cardiac cause (such as myocardial infarction, low-output failure, lethal arrhythmia), unwitnessed death and death of unknown reason, and all procedure-related deaths, involving those linked to concomitant treatment, were classified as cardiac death [14]. The MI was defined by rise of cardiac troponin (cTn) values [>5 × 99th percentile upper reference limit (URL)] in patients who have normal baseline values (≤99th percentile URL) or a increase of cTn values >20% when the baseline values are elevated and are stable or declining [15]. Pathological Q waves are defined as per amplitude, location, and depth if appear in at least two contiguous leads. A TLR was described if restenosis was within the stent or in subsequent 5 mm of distal or proximal segment. A TVR was considered when there was stenosis in any segment of the treated vessel. A ST was considered acute when it occurred within 24 hours, sub-acute when it occurred between 1 and 30 days, and late when it occurred after 30 days. A definite ST was defined by symptoms suggestive of an acute coronary syndrome and angiographic or pathologic confirmation of stent thrombosis. A probable ST was described as unexplained death in 30 days or target vessel MI without angiographic confirmation of ST. A possible ST was described as any unexplained death after 30 day [14].
Follow Up
Clinical follow up or telephonic follow up were scheduled at 30 days, 6 months and 12 months. Follow up data were obtained relating to current clinical status, prior hospitalisation and incidence of any adverse events. Further follow up is scheduled to be taken at 24 months.
Statistical Analysis
Continuous variables were presented as mean ± standard deviation and categorical variables as counts and percentages. The event free survival curve was calculated according to the Kaplan-Meier method. All data were analysed using the Statistical Package for Social Sciences (SPSS; Chicago, IL, USA) program, version 15.
Results
Baseline Demographics and Lesion Characteristics
A total of 419 patients were enrolled in the study. [Table/Fig-1] outlines the basic demographic details of patients. The study population included higher proportion of male (n=317; 75.7%) and average age was 54.1±10.2 years. The rate of hypertension was high in the enrolled patients (n=243; 58.0%). There were 134 (32.0%) diabetic patients in the study. Majority of the patients presented with stable angina (n=153; 36.5%) and non ST-elevation myocardial infarction (n=120; 28.6%). Total 491 lesions were intervened successfully with 525 stents (1.1±0.3 per lesion). The average stent length and diameter were 23.1±8.7 mm and 2.9±0.3 mm, respectively. Type B2 and C lesions accounted for 55 (11.2%) and 200 (40.7%) lesions and there were 88 (17.9%) totally occluded lesions. The lesion and procedural characteristics are detailed in [Table/Fig-2].
Baseline demographics characteristics of patients.
| Characteristics | Flexinnium (N = 419) |
|---|
| Age (mean ± SD, yrs) | 54.1 ± 10.2 |
| Male, n (%) | 317 (75.7%) |
| Diabetes Mellitus, n (%) | 134 (32.0%) |
| Hypertension, n (%) | 243 (58.0%) |
| Left Ventricular Ejection Fraction (mean ± SD, %) | 49.3 ± 9.9 |
| Smoker, n (%) | 154 (36.8%) |
| Tobacco User, n (%) | 91 (21.7%) |
| Previous Myocardial Infarction, n (%) | 180 (43.0%) |
| Previous Percutaneous Coronary Intervention, n (%) | 7 (1.7%) |
| Clinical Presentation |
| Stable Angina, n (%) | 153 (36.5%) |
| Unstable Angina, n (%) | 77 (18.4%) |
| ST-Elevation Myocardial Infarction, n (%) | 69 (16.5%) |
| Non ST-Elevation Myocardial Infarction, n (%) | 120 (28.6%) |
Lesion and procedural characteristics of patients.
| Characteristics | Patients = 419 / Lesions = 491 |
|---|
| Target Coronary Artery |
| Left main artery, n (%) | 1 (0.2%) |
| Left anterior descending artery, n (%) | 257 (52.3%) |
| Right coronary artery, n (%) | 156 (31.8%) |
| Left circumflex artery, n (%) | 77 (15.7%) |
| ACC/AHA Lesion Classification |
| A, n (%) | 57 (11.6%) |
| B1, n (%) | 179 (36.5%) |
| B2, n (%) | 55 (11.2%) |
| C, n (%) | 200 (40.7%) |
| No. of Diseased vessels |
| Single vessel disease, n (%) | 281 (67.1%) |
| Double vessel disease, n (%) | 126 (30.1%) |
| Triple vessel disease, n (%) | 12 (2.9%) |
| Total occlusion, n (%) | 88 (17.9%) |
| Total no. of stents, n | 525 |
| No. of stents per patient, (mean ± SD, mm) | 1.3 ± 0.5 |
| No. of stents per lesion, (mean ± SD, mm) | 1.1 ± 0.3 |
| Average stent length, (mean ± SD, mm) | 23.1 ± 8.7 |
| Average stent diameter, (mean ± SD, mm) | 2.9 ± 0.3 |
ACC/AHA = American College of Cardiology/American Historical Association
Clinical Outcomes
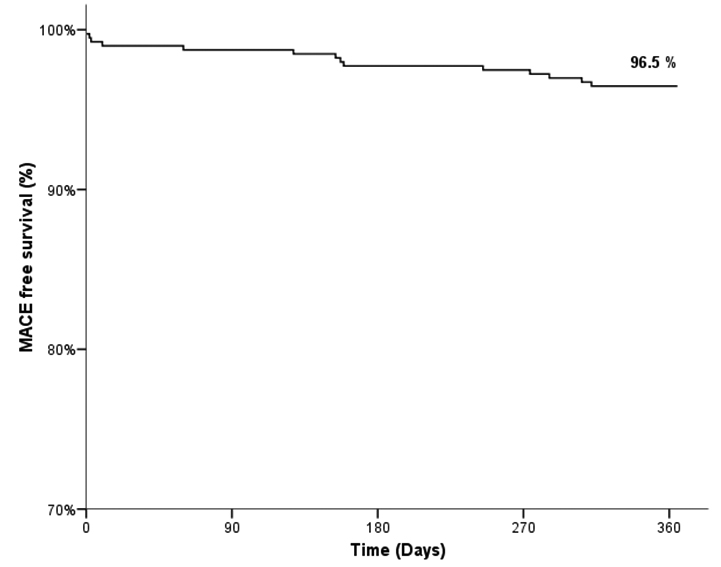
Follow up was completed in 396 (94.5%) patients at the end of 12 months. The occurrence of MACE during in-hospital stay, at 30 days, and 6 months was 5 (1.2%), 5 (1.2%), and 9 (2.2%), respectively. The MACE at 12 months was found to be 14 (3.5%). These included 9 (2.3%) cardiac deaths, 1 (0.3%) MI, 3 (0.8%) TLRs and 1 (0.3%) TVR. There was one incidence of definite ST at 12 months follow-up. [Table/Fig-3] details clinical outcomes of the study. The cumulative event free survival was found to be 96.5% by Kaplan-Meier method [Table/Fig-4].
Cumulative clinical outcomes upto 12 months.
| Variables | In-hospital N = 419; 100% | 30 days N = 419; 100% | 6 months N = 410; 97.9% | 12 months N = 396; 94.5% |
|---|
| Death | 5 (1.2%) | 5 (1.2%) | 7 (1.7%) | 11 (2.8%) |
| Cardiac, n (%) | 5 (1.2%) | 5 (1.2%) | 7 (1.7%) | 9 (2.3%) |
| Non-cardiac, n (%) | 0 (0%) | 0 (0%) | 0 (0%) | 2 (0.5%) |
| Myocardial Infarction | 0 (0%) | 0 (0%) | 0 (0%) | 1 (0.3%) |
| Q-wave, n (%) | 0 (0%) | 0 (0%) | 0 (0%) | 1 (0.3%) |
| Non Q-wave, n (%) | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) |
| Target Lesion Revascularization, n (%) | 0 (0%) | 0 (0%) | 1 (0.2%) | 3 (0.8%) |
| Target Vessel Revascularization, n (%) | 0 (0%) | 0 (0%) | 1 (0.2%) | 1 (0.3%) |
| Stent Thrombosis, n (%) | 0 (0%) | 0 (0%) | 0 (0%) | 1 (0.3%) |
| Definite, n (%) | 0 (0%) | 0 (0%) | 0 (0%) | 1 (0.3%) |
| Probable, n (%) | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) |
| Possible, n (%) | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) |
| Total MACE, n (%) | 5 (1.2%) | 5 (1.2%) | 9 (2.2%) | 14 (3.5%) |
Cumulative event-free survival rate upto 12 months.
Discussion
The Flexinnium stent has shown excellent procedural and post-procedural clinical outcomes upto 12 months follow up. The diabetic patients included in the study were 134 (32.0%). More than half (58.0%) of patients were hypertensive. Overall 266 (63.5%) patients presented with acute coronary syndromes. The type of patients included in the study are veracious representative of routine clinical practice for treatment of CAD in India. Type C lesions accounted for 200 (40.7%) lesions. Moreover, the average stent length and diameter were 23.1±8.7 mm and 2.9±0.3 mm, respectively. The primary endpoint, MACE at 12 months was found to be 3.5%. The study included real-world patients with relatively complex lesions and characteristics; still it demonstrated low MACE rates. Thus, it can be postulated that the remarkable ultrathin stent struts (60 μm); the exceptional ‘S-link’ design and the illustrious Co–Cr alloy have contributed to the splendid clinical performance.
Thinner struts have been associated with lower rates of restenosis, which has been depicted by the ISAR STEREO and ISAR STEREO II trials [16,17]. Thin struts have manifested improved stent crossing, superior trackability, and reduced periprocedural MI [18]. Additionally, the Co-Cr stents can be designed with thinner struts when compared to Stainless Steel (SS) stents owing to its high tensile properties leading to thinner struts without compromising radial strength. Previously a study had reported higher cumulative survival at six months for patients treated with Co-Cr stents than those with SS stents (log-rank p=0.03) [19]. Literature suggests that stent designs have also been responsible for the clinical outcomes. The rigid stents have resulted into development of thicker neointima at follow up when compared to flexible stents [20]. Thicker neointima consecutively results into augmented in-stent restenosis. Thus, the flexible ‘S-link’ design also contributes towards the lower MACE rates after the implantation of Flexinnium stents.
[Table/Fig-5] represents comparison of patient characteristics and clinical outcome data of some previously published studies and the present study. This study includes greater number of diabetic patients (32.0%) and longer stent length (23.1±8.7 mm) than most studies. Despite the definition of MACE was not uniform in all these studies, our comparison demonstrated favourable MACE rate in the Flexinnium study when compared to most other studies. The MACE in other studies with 12 months follow-up [7, 21-24] was higher than the present study; OMEGA study (12.8%), Polish NexGen Registry (25.2%), KARE study (8.2%), Arthos Pico Austrian Multicenter Registry (15.0%), MULTIBENE (14.5%). Moreover, Flexinnium has the lowest strut thickness among all the compared stents; this represents role of strut thickness towards the clinical outcomes after stent implantation. Based on this comparative data, it could be reasonable to infer that Flexinnium stent had depicted propitious clinical performance at 12 months follow up. In addition, recently a study had also depicted favourable outcomes after implantation of Flexinnium in unselected real-life patients with simple and complex coronary lesions [25].
Comparison of patient characteristics and clinical outcome data of some previously published studies and the present study.
| Registry/Study | Stent name | Material | Strut Thickness (μm) | No. of Patients/Lesions | Diabetic Patients (%) | Average Stent Length (mm) | Follow up (months) | MACE (%) | TLR (%) |
|---|
| Flexinnium Study | Flexinnium | L605 Co-Cr | 60 | 419/491 | 32.0 | 23.1±8.7 | 12 | 3.5 | 0.3 |
| Integrity Study [28] | S9 (Integrity) | Co-Cr | 91 | 15/30 | 53.0 | 22.2±5.8 | 16 | 21.4 | 14.3 |
| MILES Study [29] | Skylor | L605 Co-Cr | 70-95 | 1020/- | 27.1 | 19.5±10.3 | 16 | 9.9 | 5.0 |
| OMEGA Study [21] | Omega/ Rebel | Pt-Cr | 81 | 328/328 | 17.4 | 18.1±6.5 | 12 | 12.8 | 8.4 |
| Polish NexGen Registry [22] | NexGen | L605 Co-Cr | 65 | 383/- | 30.3 | 29.0 (19.0-40.0) | 12 | 25.2 | 13.7 |
| KARE Study [7] | Kaname | Co-Cr | 80 | 282/284 | 22.7 | - | 12 | 8.2 | 6.4 |
| Arthos Pico Austrian Multicenter Registry [23] | Arthos Pico | Phynox Co-Cr | 65 | 203/203 | 27.0 | 16.2±6.5 | 12 | 15.0 | - |
| MULTIBENE [24] | PRO-Kinetik | SiC-coated L605 | 60-120 | 198 | 10.2 | 15.7±3.5 | 12 | 14.5 | 11.2 |
| Driver Registry [30] | Driver | MP35N Co-Cr | 91 | 298/298 | 27.6 | - | 9 | 8.4 | 7.0 |
| MATSURI Registry [31] | Tsunami | 316L SS | 80 | 1437/1792 | 24.9 | - | 6 | 7.3 | 4.5 |
| VISION registry [32] | Vision | L605 Co-Cr | 81 | 267/267 | 23.0 | 17.2±6.3 | 6 | 6.2 | 4.3 |
| CoroFlex Blue registry [33] | CoroFlex Blue | L605 Co-Cr | 65 | 2315/2315 | 19.8 | 15.6±4.4 | 6 | 9.2 | 5.5 |
| SOLSTICE Registry [6] | SolarFlex | L605 Co-Cr | 65 | 240/292 | 29.0 | 17.5±4.8 | 6 | 5.8 | 5.0 |
Currently, the DESs have been extensively used with favourable outcomes in terms of lower rates of restenosis when compared with BMS. Nevertheless, DESs suffer a major drawback of late stent thrombosis which has been associated with a mortality rate of around 45% [26]. Moreover, the BASKET-LATE study emphasizes that DES have been allied with augmented rate of late, apparently thrombosis-related, death or non fatal MI when compared to BMS [8]. There was low rate of restenosis-related TVR after DES (4.5%) when compared to BMS (6.7%), on the other hand the rates of cardiac death (1.2% vs. 0%) and non fatal MI (4.1% vs. 1.3%) were higher after DES implantation than BMS at 7 to 18 months follow up. Likewise, the DEDICATION trial determined that long term cardiac death, presumably due to stent thrombosis, was higher in DES than in BMS (7.7% vs. 3.2%; p =0.02) [27]. Similarly, Vogt A et al., had reported that on multivariate analysis at long term follow-up in STEMI patients after the implantation of DES and BMS, DES was not found to be superior to BMS [26]. The differences in clinical outcomes of DES and BMS are now being diminished through versatile mechanical characteristics and improved deliverability of BMS. Thus, it can be proposed that as the BMS are being improved with better designs and breakthrough engineering, they have been used in parallel with DES under myriad situations.
Limitation
The present study is limited by the fact that it was an observational, non-randomized, retrospective, single-arm study without any direct concurrent comparator. In this era of drug eluting stents, the extraction of sizeable clinical data of BMS becomes difficult and takes longer time. Nevertheless, these unselected consecutive patients represent real-world practice; whereas, patients enrolled in clinical trials are carefully selected. We did not have any information on bleeding complications or antiplatelet therapy during follow-up. Despite these limitations, this study demonstrates favourable clinical outcomes that are representative of the real-world situation.
Conclusion
Our results demonstrate that the Flexinnium stent is associated with a low 12 months incidence of MACE in a wide range of real-world population. The remarkable ultrathin stent struts (60 μm); the exceptional ‘S-link’ design and the illustrious Co-Cr alloy have also contributed to the favourable clinical performance of Flexinnium. Long-term follow up would further confirm its clinical performance profile.
[1]. Mathers CD, Boerma T, Ma Fat D, Global and regional causes of deathBritish Medical Bulletin 2009 92:7-32. [Google Scholar]
[2]. Garg S, Serruys PW, Coronary stents:current statusJ Am Coll Cardiol 2010 56(10 Suppl):S1-42. [Google Scholar]
[3]. Whittaker DR, Fillinger MF, The engineering of endovascular stent technology:a reviewVasc Endovascular Surg 2006 40(2):85-94. [Google Scholar]
[4]. King SB, IIIWhy have stents replaced balloons?Underwhelming evidenceAnn Intern Med 2003 138(10):842-43. [Google Scholar]
[5]. Levine GN, Bates ER, Blankenship JC, Bailey SR, Bittl JA, Cercek B, 2011 ACCF/AHA/SCAI guideline for percutaneous coronary intervention:a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines and the Society for Cardiovascular Angiography and InterventionsJ Am Coll Cardiol 2011 58(24):e44-122. [Google Scholar]
[6]. Suttorp M, Stella P, Dens J, McKenzie J, Park K, Frambach P, Ultra-thin strut cobalt chromium bare metal stent usage in a complex real-world setting (SOLSTICE registry)Neth Heart J 2015 23(2):124-29. [Google Scholar]
[7]. Carrie D, Schaechinger V, Danzi GB, Macaya C, Zeymer U, Putnikovic B, Cobalt–Chromium KAname™ coRonary stEnt system in the treatment of patients with coronary artery disease (KARE Study)J Interv Cardiol 2014 27(5):491-99. [Google Scholar]
[8]. Pfisterer M, Brunner-La Rocca HP, Buser PT, Rickenbacher P, Hunziker P, Mueller C, Late clinical events after clopidogrel discontinuation may limit the benefit of drug-eluting stents:an observational study of drug-eluting versus bare-metal stentsJ Am Coll Cardiol 2006 48(12):2584-91. [Google Scholar]
[9]. Daemen J, Wenaweser P, Tsuchida K, Abrecht L, Vaina S, Morger C, Early and late coronary stent thrombosis of sirolimus-eluting and paclitaxel-eluting stents in routine clinical practice:data from a large two-institutional cohort studyLancet 2007 369(9562):667-78. [Google Scholar]
[10]. Rubboli A, Dewilde W, Huber K, Eeckhout E, Herzfeld I, Valencia J, The management of patients on oral anticoagulation undergoing coronary stent implantation:a survey among interventional cardiologists from eight European countriesJ Interv Cardiol 2012 25(2):163-69. [Google Scholar]
[11]. Simon C, Palmaz JC, Sprague EA, Influence of topography on endothelialization of stents:clues for new designsJ Long Term Eff Med Implants 2000 10(1):143-51. [Google Scholar]
[12]. Briguori C, Sarais C, Pagnotta P, Liistro F, Montorfano M, Chieffo A, In-stent restenosis in small coronary arteries:impact of strut thicknessJ Am Coll Cardiol 2002 40(3):403-09. [Google Scholar]
[13]. Rittersma SZ, De Winter RJ, Koch KT, Bax M, Schotborgh CE, Mulder KJ, Impact of strut thickness on late luminal loss after coronary artery stent placementAm J Cardiol 2004 93(4):477-80. [Google Scholar]
[14]. Cutlip DE, Windecker S, Mehran R, Boam A, Cohen DJ, Van Es GA, Clinical end points in coronary stent trials:A case for standardized definitionsCirculation 2007 115(17):2344-51. [Google Scholar]
[15]. Thygesen K, Alpert JS, Jaffe AS, Simoons ML, Chaitman BR, White HD, Third universal definition of myocardial infarctionCirculation 2012 60(16):1581-98. [Google Scholar]
[16]. Kastrati A, Mehilli J, Dirschinger J, Dotzer F, Schühlen H, Neumann F-J, Intracoronary stenting and angiographic results strut thickness effect on restenosis outcome (ISAR-STEREO) trialCirculation 2001 103(23):2816-21. [Google Scholar]
[17]. Ürgen Pache J, Kastrati A, Mehilli J, Schühlen H, Dotzer F, Örg Hausleiter J, Intracoronary stenting and angiographic results:strut thickness effect on restenosis outcome (ISAR-STEREO-2) trialJ Am Coll Cardiol 2003 41(8):1283-88. [Google Scholar]
[18]. Jorge C, Dubois C, Clinical utility of platinum chromium bare-metal stents in coronary heart diseaseMed Devices (Auckl) 2015 8:359 [Google Scholar]
[19]. Koh AS, Choi L, Sim L, Tan JW, Khin L, Chua TS, Comparing the use of cobalt chromium stents to stainless steel stents in primary percutaneous coronary intervention for acute myocardial infarction:a prospective registryAcute Card Care 2011 13(4):219-22. [Google Scholar]
[20]. Fontaine AB, Spigos DG, Eaton G, Dos Passos S, Christoforidis G, Khabiri H, Stent-induced intimal hyperplasia:are there fundamental differences between flexible and rigid stent designs?J Vasc Interv Radiol1994;5(5):739-44. [Google Scholar]
[21]. Wang JC, Carrié D, Masotti M, Erglis A, Mego D, Watkins MW, Primary endpoint results of the OMEGA Study:One-year clinical outcomes after implantation of a novel platinum chromium bare metal stentCardiovasc Revasc Med 2015 16(2):65-69. [Google Scholar]
[22]. Milewski K, Gąsior P, Samborski S, Buszman PP, Błachut A, Wojtaszczyk A, Evaluation of safety and efficacy of NexGen–an ultrathin strut and hybrid cell design cobalt-chromium bare metal stent implanted in a real life patient population–the Polish NexGen RegistryAdvances in Interventional Cardiology 2016 12(3):45 [Google Scholar]
[23]. Strehblow C, Gyöngyösi M, Zenker G, Wallner H, Heigert M, Siostrzonek P, Small vessel stenting with cobalt–chromium stents (Arthos Pico) in a real world settingCoron Artery Dis 2007 18(4):305-11. [Google Scholar]
[24]. Vermeersch P, Appelman Y, Horstkotte D, Richardt G, Boland J, Lalmand J, Safety and efficacy of the cobalt chromium PRO-Kinetik coronary stent system:results of the MULTIBENE studyCardiovasc Revasc Med 2012 13(6):316-20. [Google Scholar]
[25]. Rajasekhar D, Vanajakshamma V, Reddy GO, Vamsidhar A, Latheef K, Reddy PS, Twelve months clinical outcomes after percutaneous coronary intervention with bare metal stents in unselected real-life patients with coronary artery disease:results from FLEXUS StudyWorld Journal of Cardiovascular Diseases 2016 6(10):342-51. [Google Scholar]
[26]. Vogt A, Schoelmerich A, Pollner F, Schlitt M, Raaz U, Maegdefessel L, Comparison of outcome in 1809 patients treated with drug-eluting stents or bare-metal stents in a real-world settingVasc Health Risk Manag 2011 7:693-99. [Google Scholar]
[27]. Holmvang L, Kelbæk H, Kaltoft A, Thuesen L, Lassen JF, Clemmensen P, Long-term outcome after drug-eluting versus bare-metal stent implantation in patients with ST-segment elevation myocardial infarction:5 years follow-up from the randomized DEDICATION trial (Drug Elution and Distal Protection in Acute Myocardial Infarction)JACC Cardiovasc Interv 2013 6(6):548-53. [Google Scholar]
[28]. Lee SWL, Chan MP, Chan KK, Acute and 16 month outcomes of a new stent:the first in man evaluation of the medtronic S9 (integrity) stentCatheter Cardiovasc Interv 2011 78(6):898-908. [Google Scholar]
[29]. Giordano A, Polimeno M, Corcione N, Fattore L, Di Lorenzo L, Biondi-Zoccai G, Synergy between direct coronary stenting technique and use of the novel thin strut cobalt chromium Skylor™stent:the mace in follow up patients treated with skylor stent [MILES Study]Curr Cardiol Rev 2012 8(1):6-13. [Google Scholar]
[30]. Sketch MH, Ball M, Rutherford B, Popma JJ, Russell C, Kereiakes DJ, Evaluation of the Medtronic (Driver) cobalt-chromium alloy coronary stent systemAm J Cardiol 2005 95(1):8-12. [Google Scholar]
[31]. Blanchard D, Danzi GB, Urban P, Moseri M, Juergens C, Guyon P, A novel ultra-thin bare metal stent (BMS):results from a worldwide registryEuro Intervention 2007 3(2):249-55. [Google Scholar]
[32]. Kereiakes DJ, Cox DA, Hermiller JB, Midei MG, Bachinsky WB, Nukta ED, Usefulness of a cobalt chromium coronary stent alloyAm J Cardiol 2003 92(4):463-66. [Google Scholar]
[33]. Bocksch W, Pomar F, Dziarmaga M, Tresukosol D, Ismail O, Janek B, Clinical safety and efficacy of a novel thin strut cobalt–chromium coronary stent system:results of the real world Coroflex Blue RegistryCatheter Cardiovasc Interv 2010 75(1):78-85. [Google Scholar]