Self-monitoring of blood glucose is an integral component of comprehensive diabetes treatment regimen as it improves glycaemic control and reduces the risks for potential clinical complications associated with poor-glycaemic control [1-5]. Real-time data obtained through the use of the BGMS reflects the influence of physical activity and diet on blood-glucose levels and thereby motivate the patients for life-style modification [6]. Moreover, the blood glucose measurements also guide the treating physician to establish pattern of abnormal glucose levels in order to make changes in treatment regimen [7]. Hence, the use of the BGMS is widely accepted in clinical practice as a part of diabetes management [8].
As the information provided from these devices is used for making treatment decisions, the accuracy of the BGMS is prerequisite for the sustained success of diabetes treatment regimen. The accuracy of BGMS can be assessed based on the guidelines set forth by ISO 15197:2003 describes requirements of design, safety and analytical performance for BGMS. In view of advancement in technology and importance of BGMS in the treatment of diabetes, the guidelines were revised in 2013 with more stringent criteria (ISO 15197:2013). However, Klonoff and Prahalad claim that only 32% (31/98) approved BGMS met analytical accuracy criteria of ISO 15197:2013 [9].
It should be noted that analytical performance evaluations provide standardized and reproducible information for measurement quality of the BGMS when the measurements are performed by well-trained laboratory personnel [10]. However, untrained users (patients) may found difficulty in obtaining accurate results if the high-quality BGMS is difficult to handle or if the instructions for use are incomplete or unclear [10]. Hence, ISO 15197 requires user performance evaluation to confirm if intended users are able to achieve accurate measurement results with the BGMS (based upon instructions of use, without any training or assistance).
The EXIMO™ (Meril Diagnostics Pvt. Ltd., Vapi, Gujarat, India) is the BGMS which is intended for in vitro diagnostic use by diabetic patients or by healthcare professionals in a clinical environment. The EXIMO™, BGMS utilises test-strips that contains flavin adenine dinucleotide-glucose dehydrogenase (FAD-GDH) enzyme in combination with a proprietary electron mediators to measure fresh capillary or venous blood glucose levels [11]. The glucose range of EXIMO™ is 20-500 mg/dl. The objective of the present study was to assess performance of EXIMO™, as per ISO 15197:2013 section 8 user performance criteria.
Materials and Methods
This was a single center, non-randomized, post-marketing study. The study was conducted between September 2016 and November 2016 at Bombay Maternity and Surgical Hospital, Surat, Gujarat, India. The study enrolled patients of either sex, aged ≥18 years and diagnosed with diabetes mellitus. The clinical records of the patients were reviewed for confirmation of diabetes and comorbidities. Patients were excluded: 1) if they had haemophilia, bleeding disorder, or clotting problems; 2) if they had infections or skin disorders at the puncture site or any other condition which in opinion of the investigator would put the participant or study at risk. Written informed consent was obtained from all the participants before the commencement of the study. The study was approved by Institutional Review Board.
Prior to blood testing, each study participant was provided user guide and quick reference guide to learn how to use the EXIMO™ BGMS. Participants were given adequate time to practice testing with BGMS. However, none of the patient received additional training to learn self-estimation of the blood glucose using the BGMS. The patients performed fingertip blood glucose measurement using the BGMS. After capillary blood glucose estimation with the BGMS, venous blood sample from each patient was obtained (within 5 minute) by a trained health care professional. Plasma glucose concentrations of these venous blood samples were determined by the Cobas Integra 400 plus (Roche Instrument Centre, Rotkreuz, Switzerland) which served as the reference values.
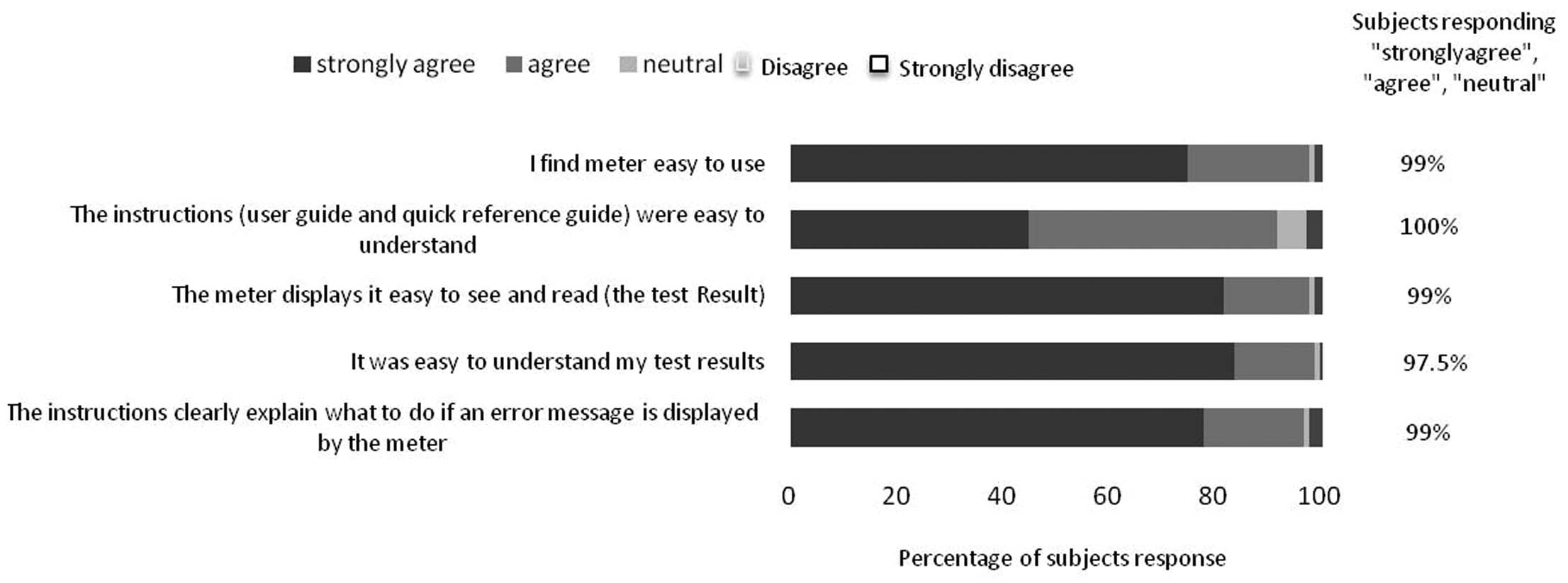
After blood glucose measurement, all the patients completed a questionnaire on the BGMS and instructions for use. Questionnaire was designed according to requirements of ISO 15197:2013(E) [12]. The questionnaire is enlisted below:
I find meter easy to use.
The instructions were easy to follow.
The test results displayed on the meter were easy to see.
It was easy to understand the test results.
The instructions clearly explain what to do if an error message is displayed on the glucose monitor.
Responses provided by the patients were scored on basis of Likert-type scale {strongly disagree (1) to strongly agree (5)}.
Statistical Analysis
The performance of the EXIMO™ BGMS was assessed as per ISO 15197:2013 section 8 criteria (≥95% of results fall within ±15 mg/dl of the reference results for sample blood glucose <100 mg/dl and ±15% of the reference results for sample blood glucose ≥100 mg/dl). Bland-Altman plot was generated to display the difference between the BGMS results and the reference results [13]. The CEG analysis was performed to assess the clinical importance of the BGMS errors [14]. The analyses were performed using a Microsoft ExcelTM VBA macro program (Microsoft Corporation, Redmond, WA, USA). We also performed SEG analyses, a novel error grid system introduced recently by several medical societies and authorities [15].
Results
A total of 1005 patients participated in the study. Average age of patients was 44.93±14.65 (Median 44 years; range 18-95 years). The demographic detail of the patients is summarized in [Table/Fig-1].
Demographic characteristics of the study subjects.
| Number of subjects | n=1005 |
|---|
| Age, Mean±SD | 44.93±14.65 |
| Number of female, n (%) | 551 (54.80%) |
| Number of male, n (%) | 454 (45.20%) |
| Educational level |
| Less than high school, n (%) | 335 (33%) |
| High school, n (%) | 171 (17%) |
| Some college or technical school, n (%) | 131 (13%) |
| Graduate degree, n (%) | 236 (24%) |
| Postgraduate degree, n (%) | 132 (13%) |
All the included patients performed fingertip puncture to measure capillary blood glucose level using the EXIMO™ system. The range of capillary blood glucose concentration as measured by the BGMS was 38 mg/dl to 482 mg/dl (median 97 mg/dl). Similarly, the range of venous blood glucose concentration as measured by reference method was 44 mg/dl to 500 mg/dl (median 96.9 mg/dl). Mean glucose levels measured by EXIMO™ and reference method were 123.06±72.02 mg/dl and 125.37±73.35 mg/dl, respectively. A total of 568 (57%) samples had glucose concentrations ≤100 mg/dl and the remaining 437 (43%) samples showed glucose concentrations >100 mg/dl.
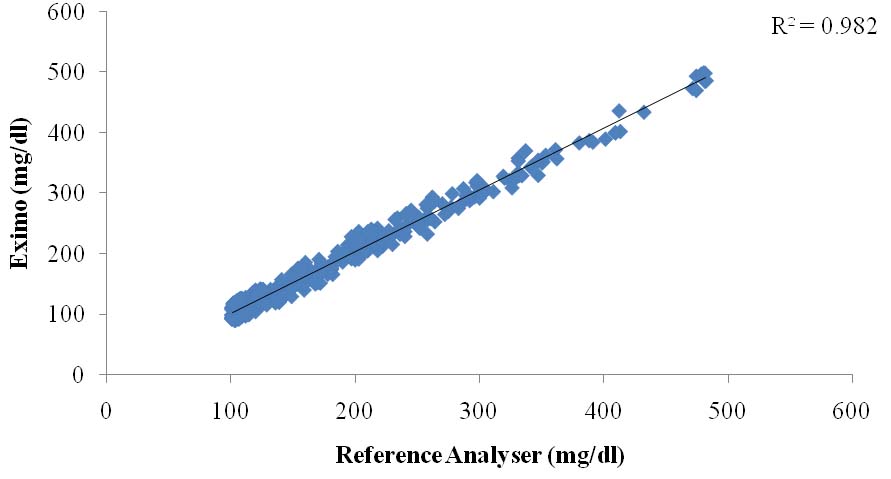
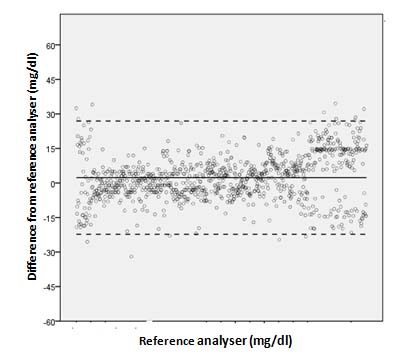
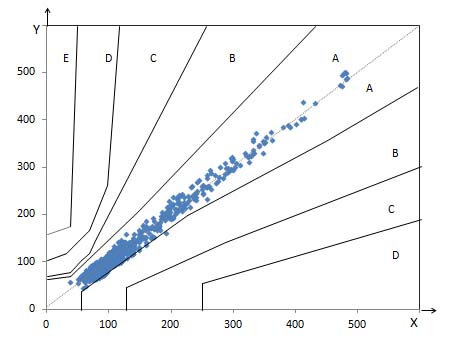
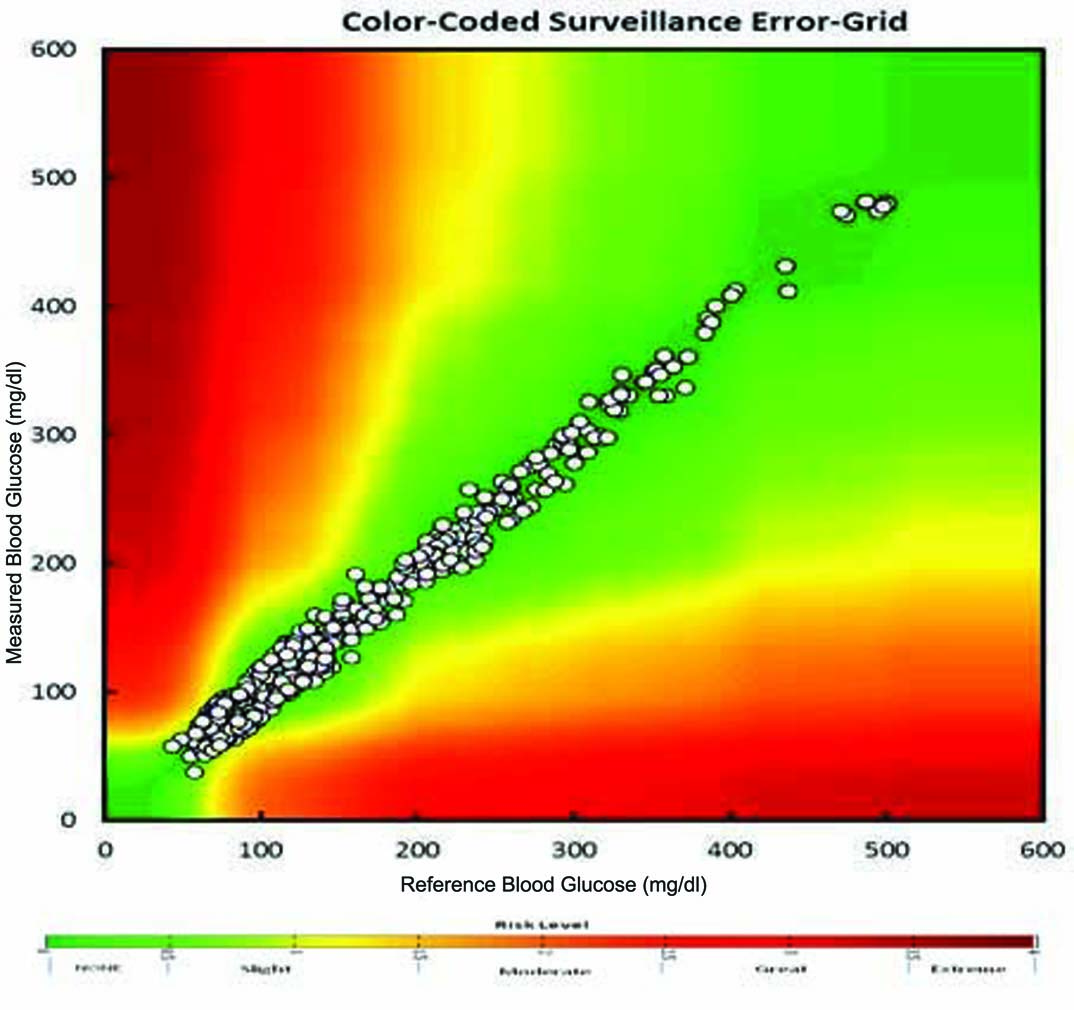
Evaluation of capillary fingertip blood glucose measurements demonstrated that 95.82% measurements fulfilled ISO 15197:2013 section 8 criteria. [Table/Fig-2] depicts summary of blood glucose measurements as per ISO 15197:2013 section 8 criteria. Regression analysis demonstrated strong agreement between BGMS measurements and reference measurements (R2=0.982) [Table/Fig-3]. The Bland-Altman plots of the difference of the BGMS measurements from reference measurements are shown in [Table/Fig-4]. The CEG analysis showed that 99.47% (n=1000) of the results fell in Zone-A and the remaining results (0.53%, n=05) fell in Zone-B which were clinically acceptable [Table/Fig-5]. The SEG analysis demonstrated that majority of the results fell within “no-risk” zone (risk score 0 to 0.5; 90.42%) [Table/Fig-6]. There was no occurrence of adverse event during study.
Summary of BGMS results as per criteria of ISO 15197:2013 section 8.
| Glucose concentration | Number of results within specified limits |
|---|
| ± 5 mg/dl | ± 10 mg/dl | ± 15 mg/dl | ± 20 mg/dl |
|---|
| < 100 mg/dl (n = 568 ) | 257 (45.24%) | 429 (75.52%) | 545 (95.95%) | 568 (100%) |
| > 100 mg/dl (n = 437) | 245 (56.06%) | 344 (78.71%) | 418 (95.65%) | 437 (100%) |
| Total(n = 1005) | 502 (49.95%) | 773 (76.91%) | 963 (95.82%) | 1005 (100%) |
Linear regression analysis of EXIMO™ measurements versus laboratory reference measurements.
Bland-Altman plot demonstrating difference of EXIMO™ measurements from reference measurements.
Parkes-Consensus Error Grid analysis of EXIMO™ measurements.
X axis denotes: reference glucose (mg/dl)
Y axis denotes: test device glucose (mg/dl)
Surveillance Error Grid analysis indicates risk levels of measurement errors.
Subject questionnaires: More than 95% of patients were strongly agree/agree with the questionnaire with respect to use of instrument, meter display, result understanding and kind of procedure after error displayed by the meter. However, approximately 90% of population agreed with understanding of reference guide or instructions. [Table/Fig-7] depicts results of the ease-of-use subject questionnaires.
Ease of use questionnaire for the use of EXIMO™ blood glucose monitoring system.
Discussion
The accuracy of BGMS is becoming increasingly important. Accurate BGMS eliminate adverse clinical and economic consequences of erroneous glucose measurement results [9]. Schnell O et al., observed reduction of BGMS errors from 20% to 15% resulted into considerable cost saving (€1.02 million and €6.03 million) due to reduction in adverse clinical consequences (1% in severe hypoglycaemia, 0.14% in A1C and 0.18% in myocardial infarction) [16].
It is critical that BGMS perform well in the hands of the intended users (people with diabetes and healthcare professionals). Recently, Hasslacher C et al., found that only fewer than half of the current BGMS fulfil accuracy requirements according to the ISO accuracy limits in a clinical setting [17]. Moreover, several studies evaluated performance of BGMS in which an experienced/trained healthcare professional performed the blood sampling [18]. Hence, the results of this study reflect mainly the technical performance of the devices. Errors (caused by patients) which are likely to be experienced in routine use may not be encountered in such studies [19]. In our study, enrolled patients themselves performed fingertip blood glucose measurement using the BGMS without any additional training (other than the user manual) to learn self-estimation of the blood glucose using the BGMS. Hence, the design of our study allowed occurrence of potential errors from all sources. Despite of this fact, the results of the study demonstrated that 95.82% of the measured glucose values fell within ±15 mg/dl or 15% of the reference method value for glucose concentration <100 mg/dl and ≥100 mg/dl, respectively. Moreover, the CEG analysis assigns the error of BGMS measurement to 1 of 5 increasing clinical risk categories i.e., Results in Zone A indicates no effects of erroneous measurement on clinical action whereas results in Zone B indicates little to no effects on clinical action [14]. In the present study, all the measurements fell either in Zone A (99.47%) or Zone B (0.53%). However, it should be noted that CEG analysis does not take into account the advancement in diabetes therapy [20]. Hence, we further performed SEG analysis which has been developed as a result of joint effort of various medical societies and authorities (Diabetes Technology Society, Food and Drug Administration, American Diabetes Association, the Endocrine Society, and the Association for the Advancement of Medical Instrumentation, as well as representatives of academia, industry and government) [15]. The SEG analysis is suitable for both type 1 and type 2 diabetes patients. It displays clinical risks on a continuous colour-coded scale and allows greater precision in quantifying low risk [15]. In our study, 90.42% results fell in “No risk” zone, 9.05% results fell in “Slight, lower risk” zone and only 0.49% results fell in “Slight, higher risk” zone. Moreover, majority of the enrolled patients felt that EXIMO™ BGMS was easy to use. Currently, several BGMS are available in the market i.e., Contour Next USB (CNU; Bayer Consumer Care, Basel, Switzerland), FreeStyle InsuLinx (FIL; Abbott, Ludwigshafen, Germany), and OneTouch Verio IQ (OT; LifeScan, High Wycombe, UK) which utilise enzymatic method in their test strips (FAD-GDH) [20,21]. Bedini JL et al., compared 3 BGMS viz. CNU, FIL and OT for Blood glucose level between reference value and meter value. Their results by CEG and SEG analyses suggest a high accuracy of the CNU compared to other 2 BGMS. For CEG analysis, 100%, 98.80% and 99.30% for CNU, FIL and OT BGMS fall into Zone A i.e., “no effect on clinical action” respectively. While, 97.04% of measurements with the FIL BGMS were within the “no risk” zone of SEG analysis [21]. In contrast, EXIMO™ fell in Zone A with 99.47% and 0.53% (Zone B) of CEG analysis and 99.47% fall into “No risk” and “Slight lower risk” zone in SEG analysis. Therefore, EXIMO™ BGMS showed similar results with other comparable BGMS.
Consequently, the results of the study assure that the intended users (healthcare professionals and patients) of the BGMS can acquire accurate blood glucose measurements in real-life settings which may guide treating physician for deciding (diabetes) treatment strategy.
Limitation
The limitation of the study includes probability of skin infections. In this study, we compared venous blood in reference laboratory method rather than fresh capillary whole blood samples. In addition, the glucose measurement by the BGMS was not performed in duplicate.
Conclusion
We conclude that the compliance of EXIMO™ with tighter criteria of ISO 15197:2013 in this post-marketing study ensures performance of the system in the hands of untrained intended users in clinical settings.
Conflict of interest: Dr. Ashok S. Thakkar and Dr. Pankaj S. Kothavade are the employees of Meril Life Sciences Private Limited, Gujarat, India and all other authors have no conflict of interest.