Laryngeal SCC has the second highest incidence of SCC of the head and neck. About 160,000 new cases of laryngeal cancer are diagnosed annually [1]. In Egypt, laryngeal cancer ranks the first among respiratory cancers (27.8%) and the tenth among all total cancers (1.7%) according to cancer registry performed by National Cancer Institute (NCI) [2].
No treatment has provided a satisfactory therapeutic outcome despite major advances in surgery and radiotherapy over the past few decades, and high mortality rate of laryngeal SCC is still reported, with a five year survival rate of 64% [3]. Thus, much work is carried out on the identification of better biologic factors that could be potential prognostic markers [4].
Dysplasia often precede laryngeal cancer; the exact mechanism by which these lesions progress to invasive carcinoma is still unknown [5].
Podoplanin is a transmembrane glycoprotein that is expressed in variety of normal tissues as renal podocytes, skeletal muscle, placenta, lung and heart, in myoepithelial cells of the breast and salivary glands and mesothelial cells [6,7].
In vascular system, podoplanin expression is totally confined to Lymphatic Endothelial Cells (LECs) that allow differentiating lymphatic vessels from blood vessels by immunohistochemical staining [8].
Podoplanin is expressed in different human cancers as SCC of the oral cavity, lung, oesophagus, uterine cervix, skin, also in dysgerminomas, granulosa cell tumours of the ovary, mesothelioma, and in several Central Nervous System (CNS) tumours [9]. In oral cancer, podoplanin has been identified as a marker of malignant development, progression and poor prognosis [10].
The recent works are concentrated on recognition of a novel molecular factor that might serve as both diagnostic and prognostic marker even at a premalignant stage. In human cancers, podoplanin expression and its correlation with tumour invasive potential raise its possible role as a diagnostic and prognostic marker for cancer [11]. So we aimed in the current work to investigate the immunohistochemical expression of podoplanin in SCC and dysplasia of the larynx to elucidate its possible role in malignant transformation and progression.
Materials and Methods
This retrospective cross-sectional study included a total of 60 archived, formalin fixed, paraffin embedded tissue blocks (40 cases of laryngeal SCC and 20 cases of dysplastic lesions) obtained through laryngoscopic biopsies and total laryngectomy specimens collected from Pathology Department, faculty of Medicine, Cairo University from “January 2014 to December 2015”. The medical records were revised and clinical data as age, gender, site, lymph node status and stage of the cancer were documented. The study took the approval of ethical committee in faculty of Medicine, Cairo University.
Each paraffin block was re-cut by rotatory microtome at five microns thickness then mounted on glass slides to be stained by Haematoxlyin and Eosin (H&E) for histopathological re-evaluation.
Histologic grading of dysplastic lesions was assessed according to WHO criteria and classified into three grades: mild, moderate and severe dysplasia [12].
Histologic grading of SCC was performed according to Broders’ classification [13].
Stage of the disease was determined according to the American Joint Committee on Cancer (AJCC) TNM system Tumour, Lymph node, Metastasis [14].
Immunohistochemistry
Paraffin embedded sections were made at 4 microns thickness on poly-L Lysine coated slides and were deparaffinized by fresh xylene, followed by rehydration in graded alcohol. Subsequently, blocking of endogenous peroxidase was done by treating the sections with peroxide block for 15 minute in room temperature, followed by antigen retrieval for 15 minute in pressure cooker. Then the sections were incubated with power block for 15 minute, and primary antipodoplanin monoclonal antibody, clone D2-40, ready to use (DakoAutostainer/ Autostainer Plus) was incubated for 40 minute. DAB chromogen was then incubated for 5-10 minute. Finally, slides were washed and haematoxylin was used as counterstain. Staining of the cell membrane and/or cytoplasm for podoplanin was considered positive expression. The lymphatic endothelial cells in the stroma positively stained for podoplanin served as an internal control.
Immunostaining for podoplanin in dysplastic lesion was scored as follows [10]:
Score (0): No staining detected in any portion of the epithelium.
Score (1): Staining confined to the basal layer of the epithelium only.
Score (2): Focal staining in one area of the basal and suprabasal layers.
Score (3): Staining in two or more areas of suprabasal layer.
Lesions with scores ≥ 2 were considered as podoplanin positive.
In laryngeal carcinomas, podoplanin immunostaining was scored as follows [11]:
Score (0): No staining detected in any portion of the tumour.
Score (1): 0-25% of the tumour cells were positively stained.
Score (2): 25-50% of the tumour cells were positively stained.
Score (3): 50-75% of the tumour cells were positively stained.
Score (4): 75-100% of the tumour cells were positively stained.
The immunostaining intensity was recorded as weak, moderate and strong in dysplastic lesions and SCC.
Statistical Analysis
The SPSS statistical software version 15 was used for statistical analysis of all data. The descriptive statistics were computed to summarize the percent for qualitative variables.
The Chi-square test was used to assess difference between qualitative variables. The different parameters were tabulated and the Pearson Chi-square test was used for analysing the expression of podoplanin among different groups. The clinicopathologic data were also elicited and compared with podoplanin. The significance was set at p < 0.05 level.
Results
Among the 20 laryngeal dysplastic lesions analysed, five cases (25%) showed mild dysplasia, six (30%) cases showed moderate dysplasia and nine cases (45%) showed severe dysplasia. Among the 40 laryngeal SCC cases analysed, three cases (7%) showed grade I tumour differentiation, 30 cases (75%) showed grade II tumour differentiation and seven cases (18%) showed grade III tumour differentiation.
The expression of podoplanin in laryngeal dysplastic lesions is summarized in [Table/Fig-1]. According to the criteria previously described, lesions with score ≥2 were classified as podoplanin positive.
Evalution of podoplanin expression in dysplastic lesions of larynx.
| Podoplanin expression | Degree of dysplasia | Total | p-value |
|---|
| Mild | Moderate | Severe |
|---|
| Negative (score 0-1) | 5 (100%) | 2 (33.33%) | 2 (25%) | 9 | 0.016** |
| Positive (score 2-3) | 0 (0.0%) | 4 (66.66%) | 7 (87.5%) | 11 |
| Total | 5 (100%) | 6 (100%) | 9 (100%) | 20 |
The positive podoplanin expression in all dysplastic lesions was strong membranous staining.
Most cases of laryngeal SCC (90%) showed positive podoplanin expression while 55% of laryngeal dysplasia cases showed positive podoplanin expression and that was statistically significant (p-value=0.002) [Table/Fig-2].
Correlation between type of the lesion and podoplanin expression.
| Podoplanin expression | Type of the lesion | Total | p-value |
|---|
| Dysplasia | Laryngeal SCC |
|---|
| Negative | 9 (45%) | 4 (10%) | 13 | 0.002** |
| Positive | 11 (55%) | 36 (90%) | 47 |
| Total | 20 (100%) | 40 (100%) | 60 |
In laryngeal SCCs, four cases (10%) showed no expression of podoplanin, nine cases (22.5%) showed score 1 expression, six cases (15%) showed score 2 expression, sixteen (40%) cases showed score 3 expression and five cases (12.5%) showed score 4 expression. Score 3 was the highest percentage for podoplanin expression in the studied cases of laryngeal SCC.
The immunostaining pattern of podoplanin differed among SCC grades. Grade I and grade II SCC showed moderate to strong membranous immunostaining. Whereas SCC grade III, frequently exhibited strong cytoplasmic and membranous immunostaining.
The relationships between podoplanin expression and the clinic-pathological variables are shown in [Table/Fig-3]. Podoplanin expression was significantly increased with depth of tumour invasion (T) (p=0.035) and disease stage (p=0.026), with all stage IV tumours showing positive podoplanin expression. Podoplanin expression also showed significant increase with advanced age and male gender. There is no significant correlation between podoplanin expression and degree of tumour differentiation, nodal metastasis and site of the tumour (p <0.05).
Clinico-pathological characteristic of the laryngeal squamous cell carcinomas and correlations with podoplanin expression.
| Parameter | No (%) | Negativepodoplaninexpression | Positivepodoplaninexpression | p |
|---|
| Age<62 Years≤62 Years | 18(45%)22(55%) | 1(5.55%)3(13.63%) | 17(94.44%)19(86.36%) | 0.011** |
| GenderMaleFemale | 39(97.5%)1(2.5%) | 4(10.3%)0(0.0%) | 35(89.7%)1(100%) | 0.006** |
| tumour siteSupraglottisGlottis | 7(17.5%)33(82.5%) | 1(14.3%)3(9.1%) | 6(85.7%)30(90.9%) | 0.304 |
| T classificationT1T2T3T4 | 1(2.5%)15(37.5%)7(17.5%)17(42.5%) | 0(0.0%)4(26.66%)0(0.0%)0(0.0%) | 1(100%)11(73.33%)7(100%)17(100%) | 0.035** |
| N classificationN0N1-N3 | 28(70%)12(30%) | 4(14.29%)0(0.0%) | 24(85.71%)12(100%) | 0.224 |
| disease stageIIIIIIIV | 1(2.5%)13(32.5%)7(17.5%)19(47.5%) | 0(0.0%)4(30.77%)0(0.0%)0(0.0%) | 1(100%)9(75%)7(100%)19(100%) | 0.026** |
| degree of differentiationWell differentiatedModerate differentiatedPoorly differentiated | 3(7.5%)30(75%)7(17.5%) | 1(33.33%)3(10%)0(0.0%) | 2(66.66%)27(90%)7(100%) | 0.274 |
The staining localization of podoplanin immunostaining varied between membranous immunostaining in grade I and II while grade III, frequently showed cytoplasmic and membranous immunostaining [Table/Fig-4].
Evaluation of podoplanin localization staining in SCC of larynx.
| Pattern of podoplanin staining | Grades of SCC lesion | Total | p-value |
|---|
| Grade I | Grade II | Grade III |
|---|
| Membranous staining | 2 (100%) | 16 (59.3%) | 1 (14.3%) | 19 | 0.041** |
| Membranous and cytoplasmic staining | 0 (0.0%) | 11 (40.7%) | 6 (85.7%) | 17 |
| Total | 2 (100%) | 27 (100%) | 7 (100%) | 36 |
Regarding the intensity of podoplanin immunostaining, all dysplastic lesion showed strong intensity, while different grades of laryngeal SCC exhibited variable intensity as weak, moderate and strong as presented in [Table/Fig-5].
Evalution of podoplanin immunostaining intensity in laryngeal SCC and dysplastic lesions.
| Type of lesion | Degree of podoplanin intensity | Total |
|---|
| Negative | Weak | Moderate | Strong |
|---|
| Dysplastic lesions:Mild dysplasiaModerate dysplasiaSevere dysplasiaTotal | 4127 | 1102 | 0000 | 04711 | 56920 |
| Laryngeal SCC:Grade IGrade IIGrade IIITotal | 1304 | 0000 | 214016 | 013720 | 330740 |
Two patterns of podoplanin immunostaining (peripheral and diffuse) were also noted in variable grades of laryngeal SCC as shown in [Table/Fig-6].
Staining pattern of podoplanin immunostaining in laryngeal SCC.
| Pattern of podoplanin staining | Grades of SCC lesion | Total |
|---|
| Grade I | Grade II | Grade III |
|---|
| Peripheral pattern | 2 | 20 | 1 | 23 |
| Diffuse pattern | 0 | 7 | 6 | 13 |
| Negative | 1 | 3 | 0 | 4 |
| Total | 3 | 30 | 7 | 40 |
Discussion
Laryngeal SCC remains a significant cause of morbidity and mortality; hence it is the second most common malignant cancer among Head and Neck Cancers (HNSCC). HNSCC involves dysregulation of multiple pathways attributed to cell cycle control, apoptosis, cellular differentiation, neovascularization, tumour invasion and spread [15].
In this work we studied podoplanin expression in a subset of SCCs and dysplastic lesions of the larynx to investigate the role of podoplanin in cancer development, progression and spread.
Sixty cases were included in this study, 40 cases of squamous cell laryngeal carcinomas and 20 cases of dysplastic lesions; their mean age was 61.53±10. Our analysis indicated a possible association between podoplanin expression and age (p-value= 0.011). Sousa SF et al., stated similar result, this can be a causal finding, but may also suggest that tumours in older people are likely to be more aggressive [16].
Regarding gender parameter and podoplanin expression, strong podoplanin expression was detected with male gender (p-value= 0.006), this was in agreement with result of Almeida AD et al., [17]. On the other hand, Kim H et al., and Seki S et al., didn’t find any correlation between podoplanin expression and male gender [18,19].
Concerning laryngeal dysplasia, nine cases showed negative podoplanin expression (score 0 and 1) (45%) [Table/Fig-7a,b]. This may be attributed to early biopsied lesions before abnormality developed or variable clonal sites from which lesion had finally developed.
Immunohistochemical analysis of podoplanin expression in laryngeal dysplastic lesions; (a) Podoplanin score 0, mild dysplasia (IHC, 10X); (b) podoplanin score 1, mild dysplasia (IHC, 20X), podoplanin score 2; (c) moderate dysplasia, podoplanin score 3 (IHC, 20X); (d) marked dysplasia, podoplanin score 3, (IHC, 20X).
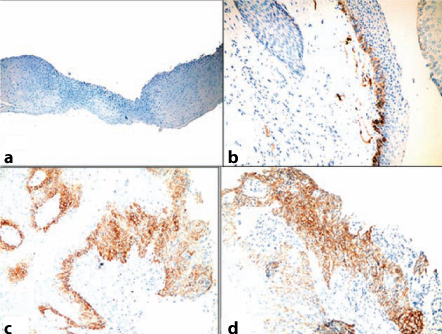
Eleven dysplastic cases (55%) showed positive podoplanin expression. These results are compatible with previous studies of Logeswari J et al., where they found that (59.4%) of cases showed positive podoplanin expression and (40.6%) of cases showed negative expression [11]. The staining pattern in dysplastic lesions was strongly membranous staining.
Among the positive cases, three cases (15%) showed podoplanin expression in the basal and suprabasal layer at one area (score 2), and eight (40%) cases expressed podoplanin in the basal and suprabasal layer at two or more areas (score 3) [Table/Fig-7c,d]. The later cases can be at possible risk of malignant transformation as stated by Kawaguchi H et al., in oral leukoplakia cases [10].
Severity of dysplasia grade is highly prone to malignant transformation, as classified by the WHO system {11% in mild–moderate and 30% in severe–carcinoma in situ (CIS)}. Furthermore, the WHO grading system is known to suffer from wide variability between pathologists. Hence, the detection of specific genes or proteins that are expressed in dysplastic lesions could be used as biomarkers for prediction of malignant transformation [20].
The expression of podoplanin beyond the basal cell layer may represent early phase of neoplastic lesions, rich in Tumour-Initiating Cells (TICs) and with a higher risk of transformation into invasive tumours [21].
Out of 40 cases of laryngeal SCC, four cases (10%) showed negative expression (score 0) [Table/Fig-8a], while 36 cases (90%) cases expressed podoplanin ranging from score 1 to 4 [Table/Fig-8b-e] and this was in agreement with previous studies of Logeswari J et al., and Raica N et al., where they found 82% of cases showed positive podoplanin expression and 18% of cases showed negative podoplanin expression [11,22].
Immunohistochemical analysis of podoplanin expression in laryngeal SCC: (a) podoplanin score 0, SCC Grade II (IHC, 20X); (b) podoplanin score 1, SCC, Grade II (IHC, 40X); (c) podoplanin score 2, SCC Grade II, (IHC, 20X); (d) podoplanin score 3, SCC Grade II (IHC, 20X); (e) podoplanin score 4, SCC Grade III, (IHC, 40X).
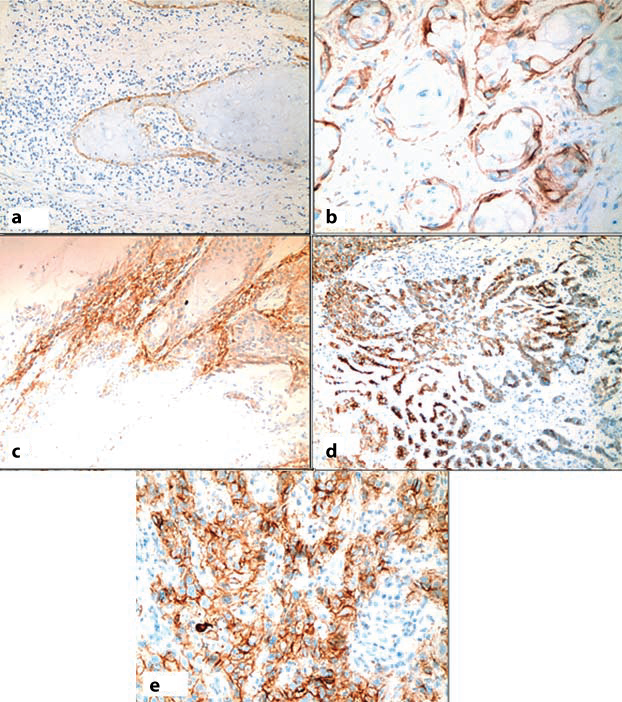
The negative expression of podoplanin in some tumours suggests that other associated factors are needed to enhanced clonal expansion of neoplastic cells. Thus, the précised role of podoplanin in tumour development is still not fully understood. It was suggested that podoplanin promote tumour invasion through reorganization of cytoskeleton of neoplastic cells, not only by combined cell migration, but also by individual cell migration after loss of E-cadherin and thus play a role in tumourgenesis [8].
In the current study, podoplanin expression showed significant positive association with SCC of the larynx compared to laryngeal dysplasia (p-value= 0.002), this agrees with result of Rodrigo JO et al., [6].
In laryngeal dysplastic lesions, there was a statistically significant correlation between the histopathological grade and expression of podoplanin (p-value= 0.016) and this coincides with results of Inoue et al., and Vicente et al., [23,24].
In laryngeal SCC, podoplanin expression in different grades was generally variable and showed no statistical significance. The staining pattern in different grades was variable. Grade I and grade II showed moderate to strong membranous immunostaining. Whereas grade III, frequently showed cytoplasmic and membranous immunostaining, these result were similar to results reported by Logeswari J et al., and Kanliada D et al., Prasad B et al., [11,25,26]. This diversion in podoplanin expression in the cytoplasm, the membrane and both of tumour cells may show enhanced propensity for tumour progression.
Podoplanin exhibited also two patterns of expression in variable grades of laryngeal SCC; diffuse and peripheral. In peripheral staining pattern, only the peripheral cells of the tumour island, exhibited podoplanin positivity. On contrast, in diffuse pattern, most of the neoplastic cells in the island displayed podoplanin positivity. Similar findings was reported by Logeswari J et al., who stated that peripheral podoplanin staining pattern may indicate lower biological aggressiveness than the diffuse pattern and that the stromal derived growth factors like epidermal growth factor, fibroblast growth factor 2, and transforming growth factor-beta might affect podoplanin expression pattern and location [11].
Our study revealed a strong association between tumour depth of invasion (T) and podoplanin expression (p-value=0.035) and these coins in agreement with result of Kreppel M et al., and Kreppel M et al., [27,28].
Furthermore, in our study podoplanin expression showed statistically significance relationship with tumour stage and these goes with result of Almeida et al., Seki et al., and Kreppel M et al., [17,19,27] and Kreppel M et al., [16,18,27]. These results suggest that podoplanin might play a role in tumour invasion and progression.
The precise function of podoplanin is not yet fully understood, but it has been proposed that podoplanin could promote tumour invasion by increasing the tumour cell mobility, cancer cell migration and invasive potential by active remodeling of the actin cytoskeleton [29].
In the current study, we didn’t found correlation between podoplanin expression and nodal metastasis (p-value= 0.654) and this was in agreement with similarly reported results by Rodrigo JO et al., Sousa SF et al., and Kanliada D et al., [6,16,25].
Limitation
The limitations of this study was not applying molecular testing for podoplanin in laryngeal SCC and dysplastic lesions due to high cost.
Conclusion
To sum up, in this study, the significant higher expression of podoplanin in laryngeal SCC cases than dysplastic lesions and its significant positive correlation with depth of tumour invasion and stage recommends podoplanin as a prognostic biologic marker in laryngeal SCC. In addition, the significant increase of podoplanin expression with higher grades of dysplasia supports its role in malignant transformation and allows us to recommend its evaluation in dysplastic lesions. Future studies should be carried out on larger scale in correlation with other prognostic factors as survival rate to confirm the precise role of podoplanin in laryngeal SCC.