Adult onset nephrotic syndrome is one of the most common presentations of primary glomerular diseases worldwide today. The salient features are proteinuria (> 3.5 gm/24 hours), hypoalbuminemia (< 2.5 gm/dL) hypercholesterolemia, oedema and hypertension [1]. The predominant glomerular diseases giving rise to adult onset nephrotic syndrome have been listed worldwide as Membranous Nephropathy (MN) [1,2], MCD [1], FSGS [1–4], MSGN [1] and MPGN [1]. Lupus nephritis [1–4]. Diabetes Mellitus [1–3] and Amyloidosis [1] are among the common systemic diseases which have been also found to be associated with nephrotic syndrome.
The renal biopsy is an invaluable method in evaluation of patients with renal disease. This procedure helps us to establish an accurate diagnosis, obtain critical information on the evaluation of prognosis of disease process and in developing a rational approach to the treatment of renal disorder [3].
MN has been reported as the most common cause of adult onset nephrotic syndrome in European and North American population [1]. However, recent studies have found the incidence of FSGS to be gradually increasing worldwide [4–7]. Limited Indian studies on spectrum of glomerular disease causing adult onset nephrotic syndrome exist till date. Among the Indian studies, MN [8] and MCD [9] have been found to be predominant primary glomerular disease although an increasing trend of FSGS has been recently observed [10–12]. MCD has been found in a few studies to be the most common overall cause of nephrotic syndrome [11,13].
The present study aimed to analyse the spectrum of glomerular diseases presenting with adult onset nephrotic syndrome in our centre over a period of three years.
Materials and Methods
This retrospective study was carried out at Ruby Hall Clinic, which is a tertiary referral centre in Pune. Adequate renal biopsies of 227 patients were analysed over a period three years from January 2007 to December 2010. Patients included for the study were between 18 to 80 years of age and presented with nephrotic syndrome. Renal biopsies with more than five glomeruli were considered adequate. Nephrotic range proteinuria was defined as proteinuria >3.5 g/1.73 m2 body surface area/day or >50 mg/kg/day. Patients <18 years of age, inadequate biopsies, allograft biopsies and incomplete immunofluoroscence data or clinical parameters were excluded from the study. All baseline clinical parameters of serum urea, serum creatinine, 24 hour urine protein, serum albumin, serum globulin, serum cholesterol and urine microscopy were recorded.
Following local anaesthesia, the renal biopsy was performed under ultrasound guidance using a 14 G Trucut biopsy gun. The biopsy material included a single core which was subjected to histopathological and Immunofluoroscence (IF) examination. Three micron sections were cut and stained with Haematoxylin and Eosin, Periodic Schiff reagent, Masson Trichrome and Jones methenamine silver for light microscopy. Additional special stains (Martius scarlet blue for fibrin) were done as and when required. IF examination was done by direct method using fluorescein isothiocyanate conjugated antibodies against immunoglobulins G, A and M and complement C3 (DAKO). IF was graded (0-3+) based on intensity of staining. The renal biopsies were analysed by two pathologists.
Due to lack of financial resources, electron microscopic examination of renal biopsies could not be carried out in this study.
All the procedures were followed were in accordance with the ethical standards of the responsible committee on human experimentation (institutional) and with the revised Helsinki Declaration of 2000.
Statistical Analysis
The data was entered using Microsoft excel sheet. Descriptive statistics was used and results were expressed as frequencies, percentages, and mean±standard deviation.
Results
A total of 227 adequate renal biopsies were analysed over a period three years from January 2007 to December 2010. Subjects included 164 (72%) males and 63 (28%) females with 63/227 (27.8%) of patients present in the 4th decade [Table/Fig-1]. Our study cohort included 36 MCD [Table/Fig-2a-c] including its variants (21 mild mesangial hypercellularity and nine diffuse mesangial hypercellularity), 30 MSGN, two FSGS [Table/Figs-3a-c], 21 IgA nephropathy, 28 MPGN (Type I), 28 MN [Table/Fig-4a-d], 19 lupus nephritis, 22 diabetic glomerulosclerosis [Table/Fig-5a-c], eight amyloidosis and 10 hypertensive nephrosclerosis.
Age distribution in study population.
| Age group | Number (%) |
|---|
| < 20 | 18 (7.9) |
| 21-30 | 58 (25.6) |
| 31-40 | 63 (27.8) |
| 41-50 | 46 (20.3) |
| 51-60 | 28 (12.3) |
| 61-70 | 11 (4.8) |
| > 70 | 3 (1.3) |
| Total | 227 |
Minimal change disease; glomeruli and tubules showing no lesions on light microscopy (20X): (a) H&E; (b) PAS; (c) Jones methenamine silver. (All images left to right)
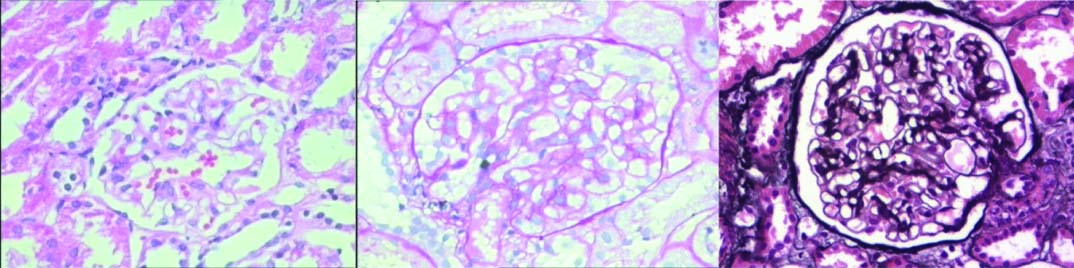
Focal and segmental glomerulosclerosis: Light microscopy shows segments of sclerosis in glomerular tufts: (a) H&E (20X); (b) PAS (20X); (c) Immunofluorescence shows IgM deposits in sclerotic tufts (20X). (All images left to right)
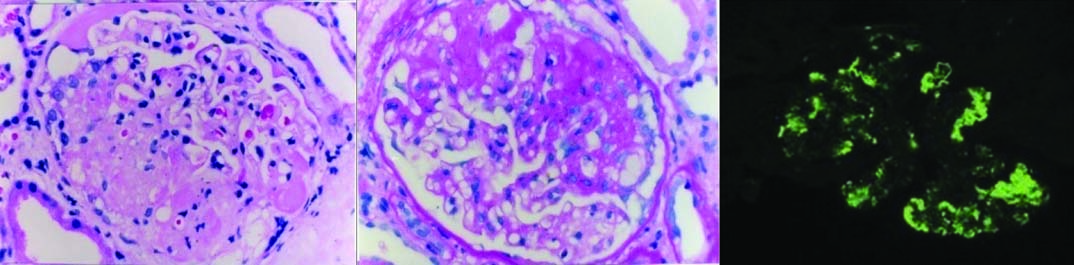
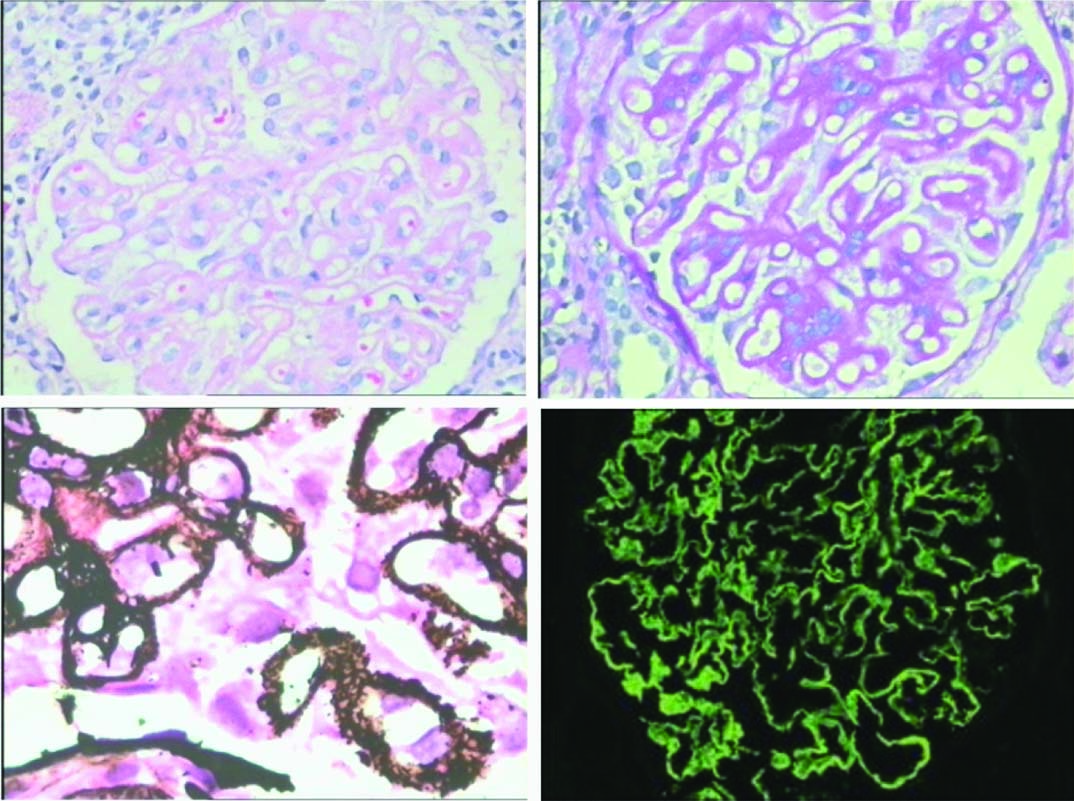
Membranous nephropathy- Light microscopy shows thickening of the capillary basement membrane: (a) H&E (20X); (b) PAS (20X); (c) Jones meth-enamine silver shows presence of epimembranous spikes (20X); (d) Immunofluores-cence reveals finely granular contiguous deposits of IgG along capillary walls (20X). (All images left to right)
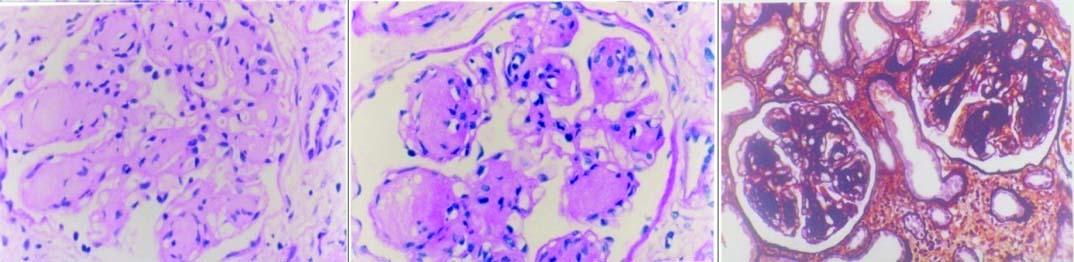
Diabetic nephropathy- light microscopy shows nodular diabetic glomerulosclerosis: (a) H&E (20X); (b) PAS (20X); (c) Jones methenamine silver (20X). (All images left to right)
Morphological spectrum of glomerular lesions with age and sex distribution: MCD and its variants i.e., mild mesangial hypercellularity and diffuse mesangial hypercellularity, 36/227 (15.9 %) was the commonest cause of adult onset nephrotic syndrome followed by MN, 28/227 (12.3%) and MPGN (Type I) 28/227 [Table/Fig-6].
Morphological lesions and their age and sex distribution.
| Diagnosis | Age (Yrs) (Ν) (%) | Sex (ν) (%) |
|---|
| < 20 | 21-30 | 31-40 | 41-50 | 51-60 | 61-70 | >70 | Total | M | F |
|---|
| 1b. Mild mesangial hypercellularity | 7 (33.3) | 4 (19.1) | 6 (28.6) | 3 (14.3) | 1 (4.76) | 0 | 0 | 21 | 4 (19.1) | 17 (80.9) |
| 1c. Diffuse mesangial hypercellularity | 1 (11.1) | 4 (44.4) | 2 (22.2) | 1 (11.1) | 0 | 1 (11.1) | 0 | 9 | 2 (22.2) | 7 (77.8) |
| 2. Mesangioproliferative GN | 3 (10) | 14 (46.7) | 9 (30) | 1 (3.33) | 3 (10) | 0 | 0 | 30 | 12 (40) | 18 (60) |
| 3. Focal segmental glomerulosclerosis | 2 (8) | 3 (12) | 8 (32) | 7 (28) | 2 (8) | 2 (8) | 1 (4) | 25 | 6 (24) | 19 (76) |
| 4. IgA nephropathy | 0 | 9 (42.9) | 7 (33.3) | 5 (23.8) | 0 | 0 | 0 | 21 | 2 (9.5) | 19 (90.5) |
| 5. Membranoproliferative GN | 3 (10.7) | 8 (28.6) | 6 (21.4) | 7 (25) | 2 (7.14) | 2 (7.14) | 0 | 28 | 6 (21.4) | 22 (78.6) |
| 6. Membranous nephropathy | 0 | 5 (17.9) | 8 (28.6) | 7 (25) | 6 (21.4) | 2 (7.14) | 0 | 28 | 8 (28.6) | 20 (71.4) |
| 7. Lupus nephritis | 2 (10.5) | 6 (31.6) | 7 (36.8) | 3 (15.8) | 1 (5.26) | 0 | 0 | 19 | 14 (73.7) | 5 (26.3) |
| 8. Diabetes glomerulosclerosis | 0 | 0 | 5 (22.7) | 5 (22.7) | 7 (31.8) | 4 (18.2) | 1 (4.55) | 22 | 3 (13.6) | 19 (86.4) |
| 9. Amyloidosis | 0 | 1 (12.5) | 1 (12.5) | 1 (12.5) | 4 (50) | 0 | 1 (12.5) | 8 | 2 (25) | 6 (75) |
| 10. Hypertensive nephrosclerosis | 0 | 4 (40) | 2 (20) | 2 (20) | 2 (20) | 0 | 0 | 10 | 2 (20) | 8 (80) |
Minimal change disease and its variants were seen most frequently in female subjects 28/36 (77.8%) and in the 4th decade, 10/36 (27.8%). Membranous nephropathy was commonly seen in the 4th decade, 8/28 (28.6%) whereas, MPGN (Type I) was seen more frequently in 3rd decade, 8/28 cases (28.6%).
Serum Creatinine: A total of 68/227 (29.86%) patients presented with abnormal creatinine levels among which 7/10 (70%) patients with hypertensive nephrosclerosis showed abnormal creatinine levels more than 1.8 mg% [Table/Fig-7]. A total of 45/227 (i.e., 19.83 %) showed raised blood urea level.
| Diagnosis | Serum creatinine | Total |
|---|
| 0.3-0.7 | 0.8-1.2 | 1.3-1.7 | 1.8-2.2 | >2.2 |
|---|
| 1a. Minimal change disease | 0 | 6 (100%) | 0 | 0 | 0 | 6 |
| 1b. Mild mesangial hypercellularity | 1 (4.8%) | 16 (76.2%) | 0 | 2 (9.5%) | 2 (9.5%) | 21 |
| 1c. Diffuse mesangial hypercellularity | 0 | 7 (77.8%) | 0 | 0 | 2 (22.2%) | 9 |
| 2. Mesangioproliferative GN | 1 (3.3%) | 22 (73.3%) | 0 | 0 | 7 (23.3%) | 30 |
| 3. Focal segmental glomerulosclerosis | 0 | 15 (60%) | 0 | 2 (8%) | 8 (32%) | 25 |
| 4. IgA nephropathy | 0 | 11 (52.4%) | 0 | 2 (9.5%) | 8 (38.1%) | 21 |
| 5. Membranoproliferative GN | 0 | 21 (75%) | 1 (3.6%) | 1 (3.6%) | 5 (17.9%) | 28 |
| 6. Membranous nephropathy | 0 | 25 (89.3%) | 0 | 1 (3.6%) | 2 (7.1%) | 28 |
| 7. Lupus nephritis | 0 | 14 (73.7%) | 0 | 1 (5.3%) | 4 (21%) | 19 |
| 8. Diabetes glomerulosclerosis | 0 | 10 (45.5%) | 0 | 5 (22.7%) | 7 (31.8%) | 22 |
| 9. Amyloidosis | 0 | 7 (87.5%) | 0 | 1 (12.5%) | 0 | 8 |
| 10. Hypertensive nephrosclerosis | 0 | 3 (30%) | 0 | 2 (20%) | 5 (50%) | 10 |
Serum and urinary protein examination: A total of 211/227 (92.9%) patients had total protein < 6.0 gm/dl. Only a single case of hypertensive nephrosclerosis had protein levels < 3.0 gm/dl [Table/Fig-8]. 12/28 (42.9%) patients and 6/28 (21.4%) patients with a diagnosis of MN showed proteinuria of 5 gm-8 gm and >8 gm in 24 hours respectively. 20/30 (66.7%) patients with MSGN showed pus cells in urine and 14/30 (46.7%) patients with MSGN presented macroscopic haematuria.
Urinary findings in study population.
| Diagnosis | Total | 24 hour urinary protein (mg)(%) | Urine sugar | Urine microscopy (h/p/f)(%) |
|---|
| 3.5-5 | 5-8 | >8 | Pus cells | RBCs | Casts |
|---|
| 1a. Minimal change disease | 6 | 2 (33.3) | 4 (66.7) | 0 | 0 | 5 (83.3) | 0 | 0 |
| 1b. Mild mesangial hypercellularity | 21 | 9 (42.9) | 12 (57.1) | 0 | 0 | 17 (80.9) | 11 (52.4) | 0 |
| 1c. Diffuse mesangial hypercellularity | 9 | 3 (33.3) | 4 (44.4) | 2 (22.2) | 0 | 5 (55.55) | 6 (66.7) | 0 |
| 2. Mesangioproliferative GN | 30 | 16 (53.3) | 9 (30) | 5 (16.7) | 0 | 20 (66.67) | 14 (46.7) | 0 |
| 3. Focal segmental glomerulosclerosis | 25 | 10 (40) | 11 (44) | 4 (16) | 0 | 15 (60) | 5 (20) | 0 |
| 4. IgA nephropathy | 21 | 10 (47.6) | 7 (33.3) | 4 (19.05) | 0 | 1 (4.76) | 1 (4.76) | 4 (19.1) |
| 5. Membranoproliferative GN | 28 | 12 (42.9) | 11 (39.3) | 5 (17.9) | 0 | 17 (60.7) | 26 (92.9) | 0 |
| 6. Membranous nephropathy | 28 | 10 (35.7) | 12 (42.9) | 6 (21.4) | 0 | 1 (3.57) | 2 (7.14) | 0 |
| 7. Lupus nephritis | 19 | 10 (52.6) | 7 (36.8) | 2 (10.5) | 0 | 2 (10.5) | 2 (10.5) | 4 (21.1) |
| 8. Diabetes glomerulosclerosis | 22 | 12 (54.5) | 9 (40.9) | 1 (4.55) | 19 (86.4) | 17 (77.3) | 5 (22.7) | 6 (27.3) |
| 9. Amyloidosis | 8 | 3 (12) | 4 (16) | 1 (4) | 0 | 0 | 0 | 0 |
| 10. Hypertensive nephrosclerosis | 10 | 7 (70) | 2 (20) | 1 (10) | 0 | 3 (30) | 1 (10) | 0 |
Correlation with serum cholesterol: Patients with Diabetic glomerulosclerosis, IgA nephropathy, lupus nephritis, hypertensive nephrosclerosis, minimal change disease and diffuse mesangial hypercellularity had raised serum cholesterol levels. A total of 66.7% of patients of MCD and its variant, diffuse mesangial hypercellularity had serum cholesterol 301-400 mg/dl. Among the systemic glomerular diseases, 90.9% of patients of diabetic glomerulosclerosis had 301-400 mg/dl of serum cholesterol [Table/Fig-9].
Morphological lesions and serum cholesterol level in study group.
| Diagnosis | Serum cholesterol level (mg/dl) (%) | Total |
|---|
| 101-200 | 201-300 | 301-400 | 401-500 |
|---|
| 1a. Minimal change disease | 0 | 0 | 4 (66.7) | 2 (33.3) | 6 |
| 1b. Mild mesangial hypercellularity | 3 (14.3) | 8 (38.1) | 8 (38.1) | 2 (9.5) | 21 |
| 1c. Diffuse mesangial hypercellularity | 1 (11.1) | 2 (22.2) | 6 (66.7) | 0 | 9 |
| 2. Mesangioproliferative GN | 26 (86.7) | 4 (13.3) | 0 | 0 | 30 |
| 3. Focal segmental glomerulosclerosis | 13 (52) | 11 (44) | 1 (4) | 0 | 25 |
| 4. IgA nephropathy | 2 (9.5) | 19 (90.5) | 0 | 0 | 21 |
| 5. Membranoproliferative GN | 20 (71.4) | 1 (3.6) | 7 (25) | 0 | 28 |
| 6. Membranous nephropathy | 13 (46.4) | 15 (53.6) | 0 | 0 | 28 |
| 7. Lupus nephritis | 5 (26.3) | 14 (73.7) | 0 | 0 | 19 |
| 8. Diabetes glomerulosclerosis | 0 | 2 (9.1) | 20 (90.9) | 0 | 22 |
| 9. Amyloidosis | 7 (87.5) | 1 (12.5) | 0 | 0 | 8 |
| 10. Hypertensive nephrosclerosis | 2 (20) | 8 (80) | 0 | 0 | 10 |
Immunofluoroscence categorisation of glomerular diseases: A total of 40 patients with MCD including its variants presented with a negative immunofluoroscene (IF). Five cases of mild mesangial hypercellularity and diffuse mesangial hypercellularity respectively showed IgM(1+) [Table/Fig-10].
Immunofluoroscence categorisation of glomerular diseases.
| Diagnosis | Total | Immunofluorescence (%) |
|---|
| IgG | IgA | IgM | C3 |
|---|
| 1a. Minimal change disease | 6 | 0 | 0 | 0 | 0 |
| 1b. Mild mesangial hypercellularity | 21 | 0 | 0 | 5 (23.8) | 0 |
| 1c. Diffuse mesangial hypercellularity | 9 | 1 (11.1) | 0 | 5 (55.55) | 1 (1.11) |
| 2. Mesangioproliferative GN | 30 | 2 (6.67) | 1 (3.33) | 6 (20) | 3 (10) |
| 3. Focal segmental glomerulosclerosis | 25 | 1 (4) | 0 | 14 (56) | 4 (16) |
| 4. IgA nephropathy | 21 | 6 (28.57) | 21 (100) | 9 (42.86) | 7 (33.33) |
| 5. Membranoproliferative GN | 28 | 20 (71.43) | 0 | 0 | 28 (100) |
| 6. Membranous nephropathy | 28 | 25 (89.29) | 0 | 0 | 10 (35.71) |
| 7. Lupus nephritis | 19 | 19 (100) | 19 (100) | 19 (100) | 19 (100) |
| 8. Diabetes glomerulosclerosis | 22 | 7 (31.82) | 0 | 7 (31.82) | 9 (40.91) |
| 9. Amyloidosis | 8 | 2 (8) | 0 | 3 (12) | 1 (4) |
| 10. Hypertensive nephrosclerosis | 10 | 2 (20) | 6 (60) | 2 (20) | 3 (30) |
Among 30 patients of MSGN, 20/30 patients had a negative IF. Five patients had shown IgM (2+) positivity in the mesangium. Two patients had shown C3 (2+) deposits in the mesangium. Patient with lupus nephritis displayed the classical full house pattern of IgG, IgM, IgA and C3 with 2-3+ intensity.
Discussion
The present study included adult patients attending the nephrology clinic with nephrotic syndrome as a presenting feature. The renal biopsy was carried out in 227 cases.
Although, nephrotic syndrome has multiple aetiologies, it is mostly due to intrinsic renal diseases [1,4,12]. In the present study, nephrotic syndrome due to primary glomerular diseases formed 74.01% of total cases. Nephrotic syndrome secondary to systemic diseases accounted for 25.99 % cases. Among the array of systemic diseases, amyloidosis, lupus nephritis and diabetic glomerulosclerosis were encountered. The majority of patients were males present commonly in the 4th decade with nephrotic syndrome.
Minimal change disease (MCD): Our study shows MCD to be the predominant cause of adult onset nephrotic syndrome in the study population. It was reported in 36 (15.8%) cases of adult patients biopsied who presented as nephrotic syndrome in our study. Its incidence in literature has been reported to be from 11.2% to 40.1%.
Among Indian studies by Date A et al., from Vellore, Agarwal SK and Dash SC from Delhi and Aggarwal HK et al., from Rohtak had reported the incidence of MCD to be 35.8%, 37% and 33.3% respectively [Table/Fig-11] [8,13,14].
Comparison of glomerular lesions among nephrotic syndrome in adults in different Indian studies [7,8,10-14].
| Authors | Sample size | Place | MPGN (type I) (%) | Minimal change Disease (%) | Membranous nephropathy (%) | Mesangioproliferative GN GN (%) | FSGS |
|---|
| Gandra D and Chennamaneni B [7] | 50 | Telangana | 7.3 | 17 | 24.4 | 12.1 | 17 |
| Date A et al., [8] | 1532 | Vellore | 13.9 | 35.8 | 13.6 | 4.5 | 18.6 |
| Rathi M et al., [10] | 364 | Chandigarh | 17.9 | 14.8 | 24.4 | 1.8 | 30.6 |
| Das U et al., [11] | 1615 | Hyderabad | 5.7 | 21.8 | 10.1 | 13.8 | 15.2 |
| Golay B et al., [12] | 410 | Kolkata | 6.6 | 27.1 | 24.6 | 8.1 | 27.4 |
| Agarwal SK and Dash SC [13] | 2250 | Delhi | 11.6 | 37 | 20 | 11.2 | 20 |
| Aggarwal HK et al., [14] | 2000 | Rohtak | 18.2 | 33.3 | 16.9 | 10 | 17.6 |
| Present study | 227 | Pune | 12.3 | 15.9 | 12.3 | 13.2 | (11.01) 25 cases |
However studies from other parts of India including Andhra Pradesh and PGI Chandigarh have shown MN and FSGS to be the predominating cause of nephrotic syndrome respectively [7,10].
International studies have shown mixed results with MCD displaying incidences of 25% [15], 40.1% [16], 44.1% [17], 15.5% [18] and 31.5% [19] followed by MN [2,20–24] and FSGS [25] to be predominating causes of adult onset nephrotic syndrome [Table/Fig-12].
Comparison of glomerular lesions among nephrotic syndrome in adults in International studies [2,15,16,18-25].
| Authors | MPGN (type I)(%) | Minimal Change Disease (%) | Membranous Neph- ropathy(%) | Mesan- giopro- liferative GN (%) | FSGS |
|---|
| Hass M et al., 19761979 [2] | 6 | 23 | 36 | - | 15 |
| Hass M et al., 19951997 [2] | 2 | 15 | 33 | - | 35 |
| Cameron JS [15] | 14 | 25 | 21 | 13 | 18 |
| Beaufils H [16] | 13 | 40.1 | 23 | 8.5 | 15.6 |
| Chang JH et al., [18] | 4 | 15.5 | 12.3 | 28.3 | 5.6 |
| Tiebosch AT et al., [19] | 20.5 | 31.5 | 4.5 | 26.5 | 9 |
| Null G and Kafle RK [20] | 21.9 | 10.2 | 42.3 | 2.2 | 8 |
| Zhou F et al., [21] | 1.5 | 25.3 | 29.5 | 20 | 6 |
| Kark RM et al., [22] | 12.2 | 11.2 | 28.6 | 6.1 | - |
| U.S. Cooperative Study [23] | 1.4 | 15.7 | 52.8 | 11.4 | - |
| Rosenberg HG [24] | 26.5 | 12 | 28 | 11.5 | 4 |
| Kazi JI et al., [25] | 4.3 | 14.8 | 26.6 | 2.5 | 39.9 |
| Present study | 12.3 | 15.9 | 12.3 | 13.2 | 11 |
Focal and segmental glomerulosclerosis (FSGS): The incidence of FSGS was reported from as low as 4% to 18% in Western literature [1,15,16]. Over the course of time, the incidence of FSGS and MN has been seen to be increasing in the world. This change has also been observed in the Indian population. Among Indian studies, the incidence of FSGS from Rathi M et al., and Golay V et al., were reported to be 30.6% and 27.4% respectively [10,12]. However, in our study FSGS was found in 25 (11.01%) cases which in comparison to other studies occupied a minor proportion of primary glomerular diseases.
Mesangioproliferative glomerulonephritis (MSGN): MSGN in our study was the second most common cause of nephrotic in 30 (13.21%) cases. A study from Korea [18] reported IgA presenting with MSGN as the most common cause of adult onset nephrotic syndrome. Similarly another study from China [21] reported the incidence of MSGN to be 20%. Our study showed 20/30 patients displayed a MSGN pattern in light microscopy with negative IF.
The pattern of mesangial proliferative GN in a renal biopsy serves to identify an important set of differential diagnosis. These include a resolving post-infectious GN, IgM nephropathy, SLE Lupus nephritis (WHO Class II), IgA nephropathy and MSGN (Idiopathic). Patients of MSGN(Idiopathic) with negative IF have been classified as some belonging to the Non IgA MSGN [8,26]. When compared to IgA nephropathy, studies have reported idiopathic non IgA MSGN to be a distinct entity [22–24] with few studies displaying a similar [23] and higher [22] progression rate of end stage renal disease when compared to IgA nephropathy. Patients with non IgA MSGN also had lower degree of haematuria, higher creatinine clearance and less glomerular and tubulointerstial lesions [24]. Waikhom R et al., from Kolkata had 19/23 (82.6%) patients in the study with non IgA [26], whereas our study had fewer 20/30 (66.7%) patients with non IgA MSGN with absent immune deposits presenting with nephrotic syndrome.
In our study, five patients of MSGN had shown IgM (2+) deposits in the mesangium. A possibility of IgM nephropathy could not be concurred without a detailed ultrastructural examination.
Two patients had shown coarsely granular C3 (2+) deposits in the mesangium. The differential diagnosis of C3 glomerulopathy presenting with nephrotic syndrome versus a recovering/partially treated atypical Post Infectious Glomerulonephritis (PIGN) was considered. A definite clinical history of infection could not be elucidated in these two cases and electron microscopy could not be performed due to financial constraints. An ultrastructural examination would have helped in resolving the diagnosis in these two cases. Subepithelial humps and deposits in the mesangial notch region [27] are more supportive of a diagnosis of PIGN. The patients were lost to follow up.
It is important to understand that being a tertiary care referral centre; we receive biopsies of previously treated patients. Partial or inadequate treatment might be the reason for deviation of classical patterns of diseases making it increasingly difficult to arrive at a conclusive aetiological diagnosis. This may account for negative IF studies in patients with MSGN.
Membranous Nephropathy (MN): The incidence of MN showed wide variation from 21.0% in a study by Cameron JS [15] to 52.8% in the series by US cooperation study [23].
MN accounted for 12.3% of patients with adult onset nephrotic syndrome. Our study also shows peak incidence in the 4th decade (28.6%) and 5th decade (25%) which is consistent with the previous studies.
In the present study, 164 patients were females and 63 were males; most of which belonged to 3rd and 4th decades i.e., 31.6 % and 36.8 % respectively which is in concordance with the previous studies [22,24].
Membranoproliferative glomerulonephritis (MPGN) (Type I): It is also one of the commonest type of lesion in our study i.e., 28 cases (28.6%) of adult patients biopsied who presented as nephrotic syndrome. In western literature, its incidence varies from 1.4% to 26.5%. The low incidence i.e., upto 1.4% in the series by US Cooperation study [23] may be because until its recognition as a separate entity, it was reported under the headings of proliferative GN and membranous GN. Significant incidence of MPGN has also been reported from Nepal (21.9%) [20], Rohtak (18.2%) [14] and Chandigarh (17.9%) [10]. In children and adults, Cameron JS [15] observed that MPGN affects both males and females equally. But in our study we had female preponderance i.e., (78.6%) over male (21.4%). MPGN type I reported to be seen in patients of all ages. Majority of cases of MPGN type II (dense deposit disease) are under the age of 20 years. In our study, MPGN type I cases were distributed in all age groups with highest incidence in 3rd decade i.e., 8 out of 28 (28.6%). We did not encounter any case of dense deposit disease in our study group.
Primary versus secondary glomerular disease: A total of 59/227 (25.99%) patients in our study had secondary systemic causes for adult onset nephrotic syndrome which is in concordance with Western and Indian literature [Table/Fig-13] [8,10-15,22,28,29].
Incidence of primary nephrotic syndrome and nephrotic syndrome secondary to systemic diseases [8,10-15,22,28,29].
| Authors | total no. of cases | Primary NS | NS due to systemic diseases |
|---|
| No. | % | No. | % |
|---|
| Date A et al., [8] | 1532 | 1276 | 83.3 | 256 | 16.67 |
| Rathi M et al., [10] | 364 | 324 | 89 | 40 | 11 |
| Das U et al., [11] | 1615 | 1278 | 79.1 | 49 | 11.9 |
| Golay V et al., [12] | 410 | 361 | 88.1 | 49 | 11.9 |
| Agarwal SK and Dash SC [13] | 2250 | 1316 | 58.5 | 934 | 41.5 |
| Aggarwal HK et al., [14] | 404 | 318 | 78.7 | 86 | 21.3 |
| Cameron JS [15] | 62 | 48 | 77.4 | 14 | 22.6 |
| Kark RM et al., [22] | 98 | 57 | 58.8 | 41 | 41.2 |
| Schreiner GE [28] | 111 | 87 | 79.4 | 34 | 20.6 |
| Blainey JD et al., [29] | 25 | 18 | 72 | 7 | 28 |
| Present Study | 227 | 168 | 74.01 | 59 | 25.99 |
Among the secondary causes of nephrotic syndrome in our study, the commonest were diabetes mellitus (9.69%) followed by lupus nephritis (8.37%). Similar prevalence of diabetes was also reported from AIIMS, New Delhi [13] concurring with our results.
Limitation
Our study included a small sample size. Hence, the results may not clearly depict the definite spectrum of primary glomerular diseases prevalent in adults presenting with nephrotic syndrome.
Electron microscopy was unavailable due to financial constraints. The use of EM would have provided a detailed histopathological analysis and thereby, may have contributed to a further change in the spectrum of glomerular diseases.
Follow up data was limited which could have provided a confirmatory diagnosis to cases presenting with diagnostic difficulties.
Conclusion
In conclusion, our study represents a comprehensive three year data of patients presenting with adult onset nephrotic syndrome attending the nephrology clinic of our hospital. Even though a recent increase in incidence of FSGS has been noted, minimal change disease from our study emerged as the predominating cause of adult onset nephrotic syndrome which is in concordance with other studies. Our study may help to understand the various aetiologies of nephrotic syndrome in this region although further studies on a larger population and different geographical locations are warranted for categorisation.