A Case of a Rapidly Enlarging Neck Mass with Airway Compromise
Libardo Rueda Prada1, Venkata Sandeep Koripalli2, Cesar Luis Merino3, Ilmana Fulger4
1 Resident, Department of Internal Medicine, St. Barnabas Hospital, Bronx, New York, USA.
2 Resident, Department of Internal Medicine, St. Barnabas Hospital, Bronx, New York, USA.
3 Resident, Department of Internal Medicine, St. Barnabas Hospital, Bronx, New York, USA.
4 Division Director, Department of Internal Medicine, St. Barnabas Hospital, Bronx, New York, USA.
NAME, ADDRESS, E-MAIL ID OF THE CORRESPONDING AUTHOR: Dr. Libardo Rueda Prada, Department of Internal Medicine, 4422 Third Ave Mills bldg 3rd, Bronx, New York, USA.
E-mail: lrueda@sbhny.org
Anaplastic Thyroid Carcinoma (ATC) is one of the most lethal tumours in humans, extremely rare in occurrence and very aggressive in nature. We hereby present a rare case of ATC with airway compromise.
A 66-year-old male, presented complaining of a non-tender anterior neck mass rapidly increasing in size associated with dry cough, hoarseness and voice changes. Imaging studies revealed a large heterogeneous centrally necrotic lobulated left thyroid mass with metastatic lymph nodes and rightward tracheal deviation. Core biopsy and immunohistochemistry stains revealed a profile consistent with ATC. Patient’s airway was compromised. Options for treatment and prognosis were discussed. Patient was discharged home with home hospice. A high index of suspicion for ATC is necessary in patients presenting with a rapidly enlarging neck mass. A prompt cytologic evaluation with metastatic work up is important to establish diagnosis. Due to its poor prognosis, an honest discussion regarding end-of-life issues must be initiated at diagnosis. Novel therapies toward genetic and epigenetic pathways have been developed, which is the basis of current clinical trials that are intended to improve clinical outcomes in the coming years.
Anaplastic thyroid carcinoma, Genetic therapy, Palliative care, Patient comfort, Prognosis, Thyroid neoplasms
Case Report
A 66-year-old male, originally from Togo, West Africa, presented to the emergency room complaining of a non-tender anterior neck mass rapidly increasing in size for the last month, associated with dry cough, hoarseness, and voice changes. He had a history of hypertension. No history of tobacco, alcohol or illicit drug used. Family history was unknown. Physical examination revealed a large 6 cm anterior neck mass, spanning bilateral thyroid lobes with surrounding palpable nodules. Ultrasound of the thyroid mass reported two complex nodules in an enlarged left lobe measuring 3.2 cm x 2.3 cm and 1.4 cm x 1.1 cm, respectively. Fine Needle Aspiration (FNA) biopsy of the mass was performed.
A work up for identifying metastatic disease was initiated. Contrast Computed Tomography (CT) of the chest revealed extensive metastatic disease within the lower neck, throughout the chest, within the left hepatic lobe and within the right T11 vertebral body. A large left and a small right likely loculated pleural effusion was also found. Left sided thoracentesis revealed a haemorrhagic pleural effusion, compatible with exudate. Cytology of the pleural fluid reported rare large atypical cells with prominent nucleoli raising the suspicion of malignancy. Patient’s informed consent was obtained.
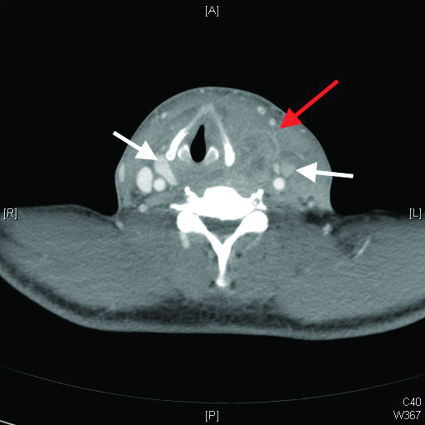
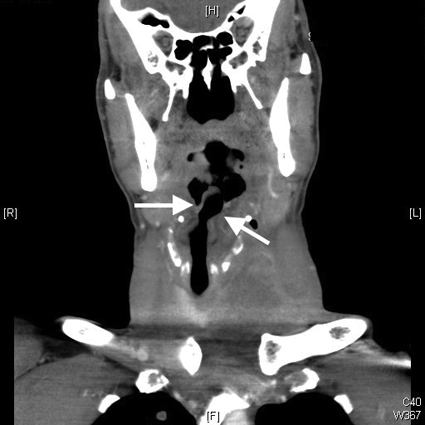
Patient was evaluated by the haematologist-oncologist who considered it highly suspicious for ATC Stage IVC based on the rapid growth of the thyroid mass and amount of metastatic disease at presentation. Contrast CT of the soft tissues of the neck was recommended to evaluate for possible airway compromise. This revealed a large heterogeneous centrally necrotic lobulated left thyroid mass measuring 6.7 cm x 4.7 cm x 8.2 cm with bilateral level III metastatic lymph nodes [Table/Fig-1], rightward tracheal deviation of approximately 1.5 cm [Table/Fig-2], ill-defined soft tissue density in the retrosternal space above the level of the brachiocephalic vein which likely represents extension of the tumour. A core biopsy of the mass was obtained for pathologic diagnosis and for BRAF, Anaplastic Lymphoma Kinase (ALK) and PIK3CA gene mutation analysis.
Axial CT scan of the neck shows heterogeneous centrally necrotic enhancing lobulated left neck mass (red arrow) and heterogeneous, likely metastatic, bilateral lymph nodes (white arrows).
Coronal CT scan of the neck shows resultant rightward tracheal deviation of approximately 1.5 cm (white arrows).
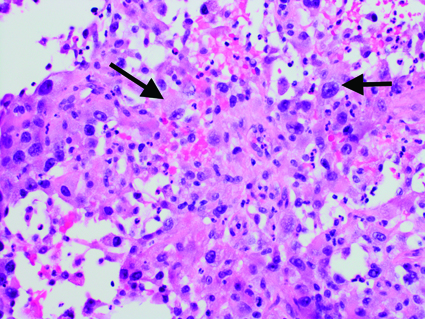
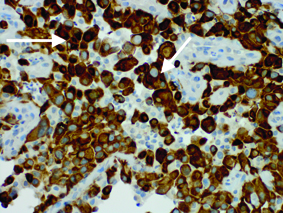
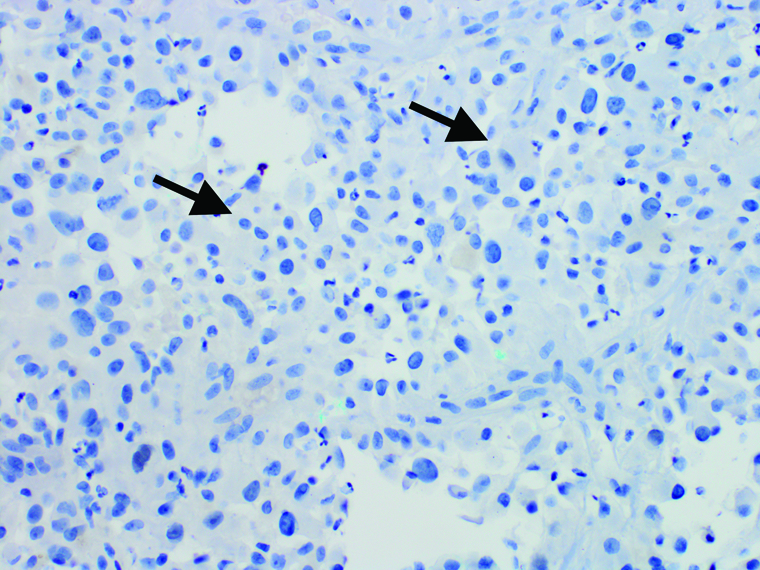
Core biopsy revealed large squamoid pleomorphic giant cells with hyperchromatic nuclei distributed in necrotic debris and along thin fibrous septae [Table/Fig-3]. Immunohistochemistry (IHC) stains revealed tumour cells positive for pan-cytokeratin [Table/Fig-4], and nuclear positivity for p53 [Table/Fig-5] while showing no expression for Thyroid Transcription Factor-1 (TTF-1) or CD45 [Table/Fig-6], an immunoprofile consistent with ATC.
Thyroid mass core biopsy. Hematoxylin-eosin stain, original magnification x400. Large squamoid pleomorphic giant cells with hyperchromatic nuclei distributed in necrotic debris and along thin fibrous septae (black arrows).
Thyroid mass core biopsy. Cytokeratin IHC stain, original magnification x400. Tumor cells positive for pan-cytokeratin (white arrows).
Thyroid mass core biopsy. P53 IHC stain, original magnification x400. Tumor cells positive for p-53 staining (black arrow).
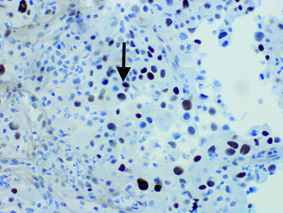
Thyroid mass core biopsy. Thyroid transcription factor-1 IHC stain, original magnification x400. Tumor cells negative for TTF-1 staining (black arrows).
During hospital stay, patient developed inspiratory stridor with airway compromise. Options for treatment and prognosis were discussed with the patient. He refused aggressive treatment interventions. After palliative care evaluation, patient decided to receive only comfort care measures. He was discharged home with home hospice.
Discussion
Thyroid cancer is the eighth most common cancer among all adults and the fifth most common cancer in women in the United States. The incidence of thyroid cancer has been increasing at the rate of 2.1% per year since 2009 with three-fold higher incidence in women than in men [1]. Thyroid cancers can be broadly classified as follicular derived and neuro-endocrine C-cell derived. Follicular derived thyroid cancers can be further divided into differentiated (which includes follicular, medullary and Hurthle cell) and ATC.
ATC is one of the most lethal tumours in humans, extremely rare in occurrence, but very aggressive in nature with common distant metastases at the time of diagnosis and a mean survival time of usually less than six months from diagnosis [2,3]. ATC accounts for less than 1% of all cases of thyroid carcinomas in the US [1]. More than 90% of the patients are older than 50 years, with a mean age of 65 years at diagnosis and a higher incidence in women [1]. A high index of suspicion is necessary in patients presenting with a rapidly enlarging neck mass, usually greater than 5 cm in diameter, fixed, hard in consistency and associated with symptoms such as hoarseness, dysphagia, shortness of breath, and cough. The median duration of symptoms before diagnosis is 1.5 months. Regional or distant spread is very common at presentation with few cases confined to the thyroid [4]. Findings of local extension include stridor, tracheal deviation, and vocal cord paralysis due to compression or invasion of the trachea as seen in this patient. This should prompt an immediate cytologic examination of the mass by FNA or surgical biopsy, with metastatic work up to establish diagnosis. Histologic pattern included a mixed morphology with giant cells, spindle cells, and squamoid cells. High mitotic activity and areas of necrosis are common. IHC staining patterns typically seen in ATC are p53 and pankeratin (AE1/AE3). Other markers such as TTF-1, PAX-8 are not typically seen [2,3]. Most common site of distant metastasis is lung, followed by bone and brain.
Treatment options depend on the stage at presentation. The Ameri-can Joint Committee on Cancer classifies all ATC as T4 and Stage IV tumours because of their aggressive behaviour. T4a is intra thyroidal tumour and surgically resectable. T4b is extra thyroidal and surgically unresectable. Stage IV is subdivided in three stages depending on the size of the thyroid tumour, and the absence or presence of nodal or distant spread. Surgical treatment is the best approach if the tumour is intra thyroidal. Unfortunately, most of the tumours are unresectable at the time of diagnosis due to the size and invasion into the surrounding structures. When the tumour is extra thyroidal, a surgical approach is controversial as the aggressive nature of this tumour results in a high rate of local recurrence. For localized tumours, combined modality with surgical debulking, and neoadjuvant or adjuvant radiotherapy and chemotherapy may prevent death from local airway obstruction and at best may slightly prolong survival. If the patient opts for a curative treatment, there is no evidence that any treatment strategy reduces tumour-specific mortality beyond a few case reports in literature review with successful remission with multi-modality treatment approach including surgical resection, radiotherapy and chemotherapy beyond the rare patient cured by surgical resection, and it is not clear that survival is prolonged by therapy other than palliative treatments [5]. An honest discussion regarding end-of-life issues and comfort measures must be initiated by palliative care at diagnosis. Patients presenting with stage IVC should be regarded as candidates for hospice care if they desire no further treatment or enrollment in a clinical trial.
Novel targeted therapeutics toward genetic and epigenetic pathways that drive ATC have been developed based on the understanding of the genomic and molecular background of this malignancy. Genetic alterations or mutations associated with ATC are most often associated with dysfunction in the ERK1/2-MEK1/2 and PI3K-AKT signaling pathways. The mutation BRAFV600E occurs in about 38% of ATC [6] and has been the focus of ongoing research. This point mutation leaves BRAF, a kinase involved in ERK1/2-MEK1/2 signaling, constitutively active and able to induce changes in the tumour microenvironment that promote tumour invasion and metastasis. Multiple mutations are involved in increased PI3K-AKT signaling pathway activity such as PIK3CA mutation, PTEN inactivation and RAS mutation [6]. Other mutations might cause the activation or inactivation of both signaling pathways. Mutations in ALK gene result in increased tyrosine kinase activity leading to activation of ERK1/2-MEK1/2 and PI3K-AKT signaling pathways [7]. On the other hand, RAS protein activator like 1 (RASAL1) inhibits both pathways and thyroid cancer growth, which supports the hypothesis that RASAL1 is a true tumour suppressor gene in thyroid cancers. The mutation of the TP53 gene causes inactivation of apoptosis and cell cycle progression in ATC [6].
There are two novel therapeutic approaches available to patients with ATC [8]. The first is a molecular targeted therapy approach based on patient mutation data. Several trials with BRAF inhibitors (such as Vemurafenib and Dabrafenib) and the combination of BRAF inhibitors with MEK inhibitors (such as Trametinib and Cobimetinib) in BRAFV600E mutated tumours are currently under development. One of these trials reported variable response to BRAF-targeted therapy possibly due to limited patient cohort [9]. Other trials are evaluating the efficacy of treatments directed against HRAS (Tipifarnib - NCT02383927) and ALK (Ceritinib - NCT02289144) mutations. The second approach is a microenvironmental-targeted approach for patients without targetable mutations where a Vascular Endothelial Growth Factor (VEGFR) inhibitor (Lenvatinib - NCT 02657369) has been used [10]. Further therapies aiming to clarify the oncogenic role of other important mutations such as TP53, PIK3CA, HRAS and PTEN mutations are under development. Due to its cost, molecular testing is not sustainable in routine clinical practice and by now it remains limited to the clinical trial setting to assist in treatment selection and future trial design.
Conclusion
ATC is a lethal tumour with lack of any effective treatment. A multidisciplinary approach at the time of diagnosis is essential to establish the optimal treatment plan. However, considering this entity’s poor prognosis, an early discussion about end-of-life planning to optimize patient’s quality of life remains paramount. Continuing research will be crucial to optimize targeted therapy approaches in patients with ATC and decrease its high mortality rate.
[1]. The Surveillance. Epidemiology, and End Results (SEER) Program of the National Cancer Institute. Available at: https://seer.cancer.gov/. Accessed December 2, 2016 [Google Scholar]
[2]. Smallridge RC, Ain KB, Asa SL, Bible KC, Brierley JD, Burman KD, American Thyroid Association guidelines for management of patients with anaplastic thyroid cancerThyroid 2012 22(11):1104-39. [Google Scholar]
[3]. Are C, Shaha AR, Anaplastic thyroid carcinoma: biology, pathogenesis, prognostic factors and treatment approachesAnn Surg Oncol 2006 13(4):453-64. [Google Scholar]
[4]. Tennvall J, Lundell G, Wahlberg P, Bergenfelz A, Grimelius L, Akerman M, Anaplastic thyroid carcinoma: three protocols combining doxorubicin, hyperfractionated radiotherapy and surgeryBr J Cancer 2002 86(12):1848-53. [Google Scholar]
[5]. McIver B, Hay ID, Giuffrida DF, Dvorak CE, Grant CS, Thompson GB, Anaplastic thyroid carcinoma: A 50-year experience at a single institutionSurgery 2001 130(6):1028-34. [Google Scholar]
[6]. Ricarte-Filho JC, Ryder M, Chitale DA, Rivera M, Heguy A, Ladanyi M, Mutational profile of advanced primary and metastatic radioactive iodine-refractory thyroid cancers reveals distinct pathogenetic roles for BRAF, PIK3CA, and AKT1Cancer Res 2009 69:4885-93. [Google Scholar]
[7]. Murugan AK, Xing M, Anaplastic thyroid cancers harbor novel oncogenic mutations of the ALK geneCancer Res 2011 71(13):4403-11. [Google Scholar]
[8]. Sandulache VC, Williams M, Lai S, Lu C, William WN, Busaidy NL, Real-time genomic characterization utilizing circulating cell-free DNA in patients with anaplastic thyroid carcinomaThyroid 2017 27(1):81-87. [Google Scholar]
[9]. Hyman DM, Puzanov I, Subbiah V, Faris JE, Chau I, Blay J, Vemurafenib in multiple nonmelanoma cancers with BRAF V600 mutationsN Engl J Med 2015 373(8):726-36. [Google Scholar]
[10]. Takahashi S, Tahara M, Kiyota N, Yamazaki T, Chayahara N, Nakano K, Phase II study of lenvatinib, a multitargeted tyrosine kinase inhibitor, in patients with all histologic subtypes of advanced thyroid cancer (differentiated, medullary, and anaplastic)Ann Oncol 2013 24(suppl 4):iv340-56. [Google Scholar]