Pharmacovigilance (PV) is the pharmacological science relating to the collection, detection, assessment, monitoring and prevention of adverse effects or any other drug-related problem [1]. It describes the process for monitoring and evaluating ADRs. It is a key component of effective drug regulation systems, clinical practice and public health programs.
Bipolar disorder is a chronic, debilitating psychiatric disorder. First-line drugs for management of bipolar disorder are mood stabilizers and atypical antipsychotics. Adjunctive medications such as anxiolytics and antidepressants are also regularly used [4]. All the effective drugs used for the treatment of bipolar disorder, no matter how competently used, may cause adverse reactions. Thus, a continuous monitoring of ADRs during post marketing phase is essential. Early detection of drug toxicity helps in timely treatment of the patient, improve compliance and decrease cost of therapy. In India, there is a scarcity of information related to ADRs occurring due to psychotropic drugs.
So, with this background, the principal aim of this prospective study was to determine the pattern of ADRs occurring in the patients of bipolar disorder coming to Psychiatry Outpatient Department (OPD) of New Civil Hospital, Surat, Gujarat, India, which is a tertiary care teaching hospital attached with Government Medical College. The ADRs were also assessed for their causality, severity and preventability.
Materials and Methods
The study was a prospective and observational study carried out in the Psychiatry OPD of New Civil Hospital, Surat for a period of 15 months after getting approval from Institutional Ethical Committee. The ethical clearance registered number is 6404/14. Patients of all ages and either gender diagnosed with bipolar disorder according to International Classification of Diseases (ICD-10) criteria and receiving pharmacotherapy from the Psychiatry OPD were included and those patients receiving pharmacotherapy for other medical or psychiatric disorders concurrently, not willing to give informed consent were excluded out from the study.
Study Procedure
Patients of both gender and all ages diagnosed with bipolar disorder and receiving treatment from psychiatry OPD were recruited for the study. The attending doctors and the nursing staff were briefed and motivated for identification and reporting of ADRs by conducting a training session in pharmacovigilance. The data was collected from interviewing the patient and their attendants as well as from patient’s case file maintained in the department of Psychiatry; and recorded in a predesigned, pre-approved patient data sheet.
The demographic details of the patient, relevant medical history and details of the treatment given were duly recorded. ADRs were confirmed after consultation with the psychiatrist and simultaneously recorded in detail for each patient. Relevant laboratory investigation values were also noted. Patients were also interviewed for any newly developed ADR when they came for their regular follow up visits.
UKU side effect rating scale [5] and AIMS [6] were used for documenting ADRs and tardive dyskinesia, respectively. Edwards and Aronson definition was used for labelling an adverse event as ADR [7].
The scoring sheet of UKU scale includes 48 items. Each item is defined by means of a 4-point-scale (0-1-2-3). As a general rule, degree 0 refers to the “normal” (the average of healthy people). Degrees “1”, “2” and “3” indicate that a symptom is present to a mild, moderate or severe degree, respectively.
The AIMS test has a total of 12 items rating involuntary movements of seven areas of the patient’s body: face, lips, jaw, tongue, upper extremities, lower extremities and trunk. These items are rated on a five-point scale of severity from 0–4. The scale is rated from 0 (none), 1 (minimal), 2 (mild), 3 (moderate) and 4 (severe).
The documented ADRs were assessed for causality, severity and preventability. Causality assessment which determines the causal relationship of a suspected drug to the ADR in question was done using both WHO-Uppsala Monitoring Centre (WHO-UMC) causality assessment scale [8] and Naranjo’s algorithm [9]. WHO-UMC scale divides causality of an ADR into six categories: “certain”; “probable”; “possible”; “unlikely”; “conditional/unclassified”; and “unassessable/unclassifiable”. Naranjo’s algorithm is a questionnaire which consists of 10 objective questions to assess the causal relationship between the ADR and the suspect drug. There are three options as answers to each question – ‘yes’, ‘no’ and ‘do not know’, which are assigned definite mathematical values (-1, 0, +1, +2) to calculate the total score. The causality is then classified based on the total score as “definite (≥ 9)”; “probable (5-8)”; “possible (1-4)”; and “doubtful (0)”.
Modified Hartwig and Siegel scale [10] was used to assess the severity and Modified Schumock and Thornton scale [11] to assess the preventability of the reported ADRs. The modified Hartwig and Siegel scale classifies severity of ADR as “mild,” “moderate,” and “severe”. The modified Schumock and Thornton scale categorizes the preventability of an ADR into “definitely preventable”, “probably preventable” and “not preventable”.
Statistical Analysis
The data was entered and analysed using Microsoft Office Excel 2010. Presentation of demographics and other numerical data was done using Descriptive statistics (Percentage, Mean±Standard Deviation, Tables and Graphs).
Association between variables was assessed using Chi-Square test and Fisher’s-exact test (Software used – Open Epi, version 2.3). A p-value < 0.05 was considered significant.
Results
A total of 207 patients diagnosed with bipolar disorder were interviewed and 180 patients were included in the study based on inclusion and exclusion criteria.
Demographic Data
The interview sample comprised of 65.56% (n=118) males and 34.44% (n=62) females with age ranging from 16 to 75 years with mean age being 37.94±12.85 years. Maximum patients belonged to 31-40 years age group (33.33%, n=60). The mean weight of the study population was 58.63±9.07 kg.
The diagnoses of patients at the time of examination are given in [Table/Fig-1].
Distribution of mood disorders (n = 180)
| Diagnosis | % of patients |
|---|
| Bipolar I Mood Disorder | 92.78 (n = 167) |
| Bipolar II Mood Disorder | 6.11 (n = 11) |
| Substance-induced bipolar | 1.11 (n = 2) |
Distribution of ADRs
Out of 180 patients, 175 (97.22%) patients developed one or more ADRs. The percentage of patients developing ADRs was slightly more in males (97.45%, n=115) as compared to females (96.77%, n=60); which was not statistically significant (Fisher’s-exact test – p > 0.05).
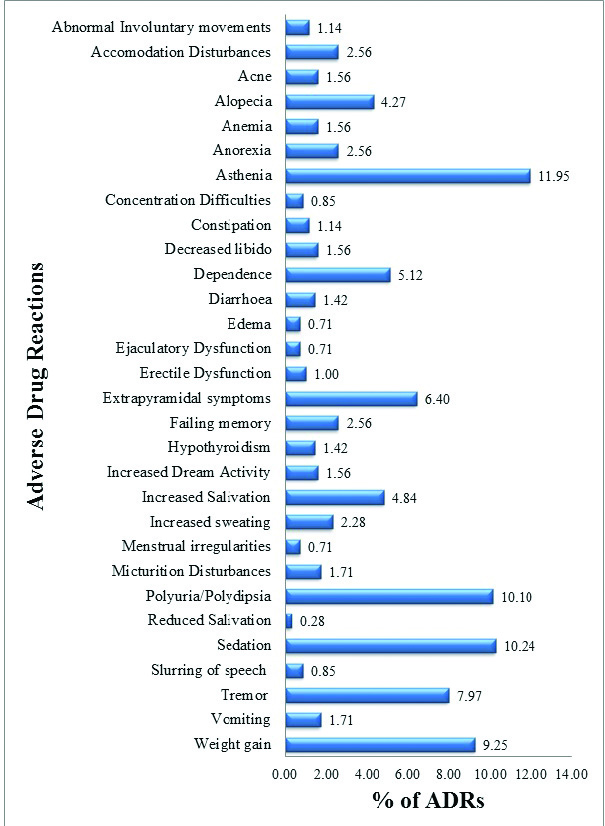
The most common ADR observed was asthenia comprising 11.95% (n=84) of total ADRs. The other frequently seen ADRs included sedation (n=72), polyuria/polydipsia (n=71), weight gain (n=65), tremors (n=56) and extra pyramidal side effects (n=45) as depicted in [Table/Fig-2].
Pattern of adverse drug reactions. (n = 703).
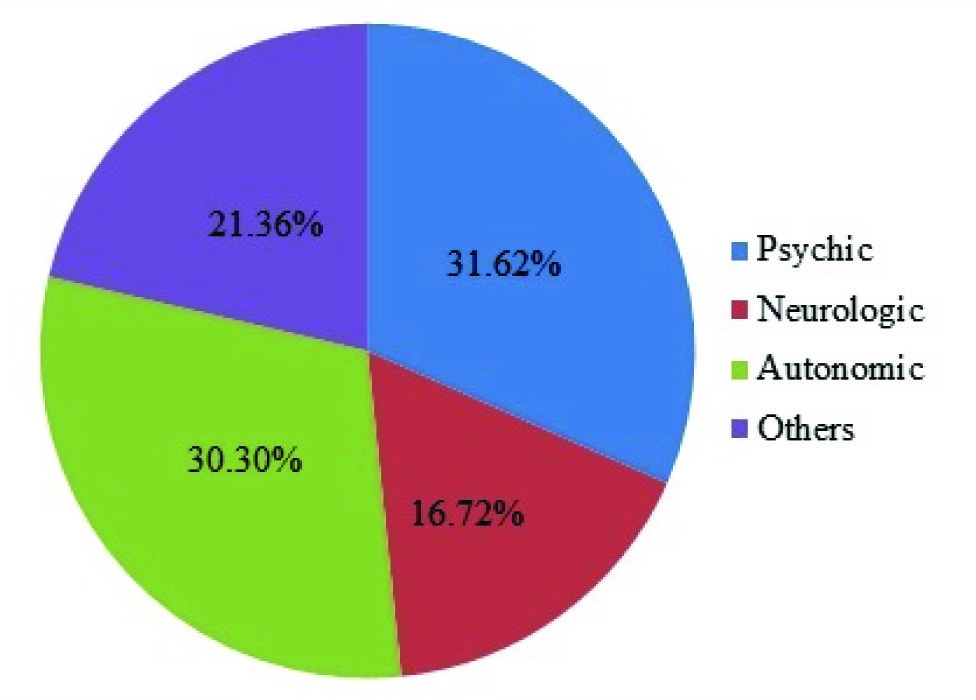
According to the UKU side effect rating scale, ADRs belonging to the group of psychic side effects were most common shown in [Table/Fig-3]. Most of the ADRs belonged to Degree 1 (n=371) according to the degrees specified in UKU scale [Table/Fig-4].
Distribution of adverse drug reactions according to UKU scale. (n =604*). (* Acne, Anaemia, Abnormal involuntary movements, Alopecia, Anorexia, Oedema, Hypothyroidism and Slurring of speech were not included in this evaluation as they are not a part of standard UKU side effect rating scale.)
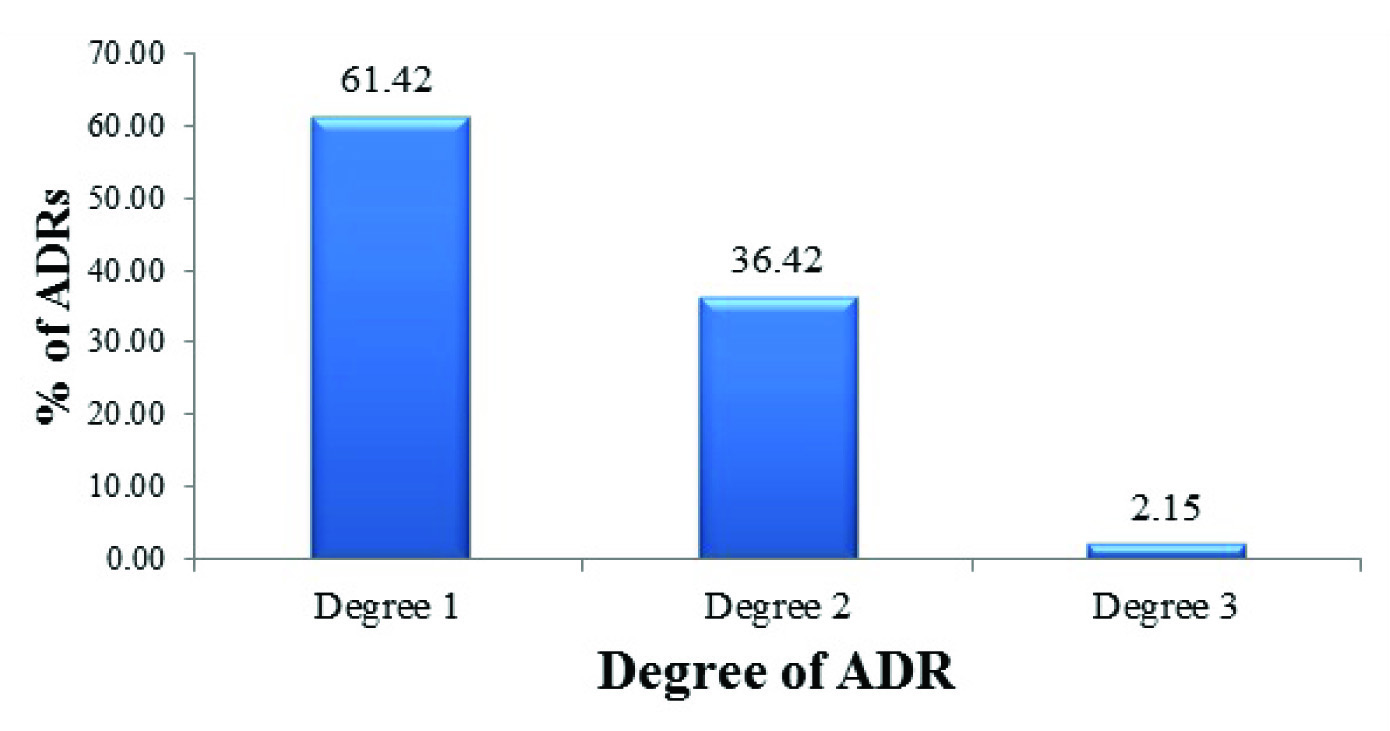
Degree of Adverse drug reactions according to UKU scale. (n = 604*). (*Acne, Anaemia, Abnormal involuntary movements, Alopecia, Anorexia, Oedema, Hypothyroidism and Slurring of speech were not included in this evaluation as they are not a part of standard UKU side effect rating scale.)
[Table/Fig-5] gives the various system organ class affected by ADRs according to the WHO – Adverse Reaction Terminology. Psychiatric disorders (21.91%, n=154) were the most common system organ class affected.
Organ systems affected due to adverse drug reactions according to the WHO adverse reaction terminology.
| Sr. No. | SOC (WHO-ART SOC Code) | Percentage of ADRs (n =703) | Adverse Drug Reactions (number of patients affected) |
|---|
| 1. | Psychiatric disorders (0500) | 21.91 (n = 154) | Sedation (72), Dependence (36), Failing memory (18), Increased dream activity (11), Decreased libido (11), Concentration difficulties (6) |
| 2. | Neurological disorders (0400) | 16.36 (n = 115) | Extrapyramidal symptoms (45), Tremors (56), Abnormal involuntary movements (8), Slurring of speech (6) |
| 3. | Body as a whole – general disorders (1810) | 14.94 (n = 105) | Asthenia (84), Increased sweating (16), Edema (5) |
| 4. | Urinary tract disorders (1300) | 11.81 (n = 83) | Polyuria/Polydipsia (71), Micturition disturbances (12) |
| 5. | Metabolic and nutritional disorders (0800) | 11.81 (n = 83) | Weight gain (65), Anorexia (18) |
| 6. | Gastrointestinal disorders (0600) | 9.39 (n = 66) | Increased salivation (34), Vomiting (12), Diarrhoea (10), Constipation (8), Reduced salivation (2) |
| 7. | Skin and appendages disorders (0100) | 5.83 (n = 41) | Alopecia (30), Acne (11) |
| 8. | Vision disorders (0431) | 2.56 (n = 18) | Accomodation disturbances (18) |
| 9. | Reproductive disorders (1400) | 2.42 (n = 17) | Erectile dysfunction (7), Ejaculatory dysfunction (5), Menstrual irregularities (5) |
| 10. | Blood disorders (1200) | 1.56 (n = 11) | Anemia (11) |
| 11. | Endocrine disorders (0900) | 1.42 (n = 10) | Hypothyroidism (10) |
Suspected Drugs
Mood stabilizers (59.46%, n=418) were the most common group of drugs associated with ADRs followed by atypical antipsychotics (25.04%, n=176). Among the mood stabilizers, lithium was responsible for 61% (n=255) ADRs followed by valproate (36.60%, n=153) and carbamazepine (2.39%, n=10). Among atypical antipsychotics, olanzapine (48.76%, n=98) was most commonly associated with ADRs followed by risperidone (25.04%, n=40). The remaining 18.91% (n=38) ADRs were associated with clozapine, quetiapine, aripiprazole and ziprasidone. Adjuvant medications such as antidepressants and sedative-hypnotics which are commonly used in bipolar disorder patients were associated with 15.50% (n=109) ADRs.
The list of drugs implicated in the most common ADRs is given in [Table/Fig-6].
Suspected medications associated with the most frequently seen adverse drug reactions.
| Sr. No. | Adverse drug reaction | Drugs implicated in decreasing order of frequency |
|---|
| 1. | Asthenia | Lithium, Valproate |
| 2. | Sedation | Olanzapine, Clonazepam, Diazepam, Lorazepam |
| 3. | Polyuria/Polydipsia | Lithium |
| 4. | Weight gain | Olanzapine, Valproate, Clozapine and Lithium |
| 5. | Tremor | Lithium, Valproate |
| 6. | Extrapyramidal symptoms | Risperidone, Olanzapine, Aripiprazole, Quetiapine, Ziprasidone |
Causality, Severity and Preventability Assessment of ADRs
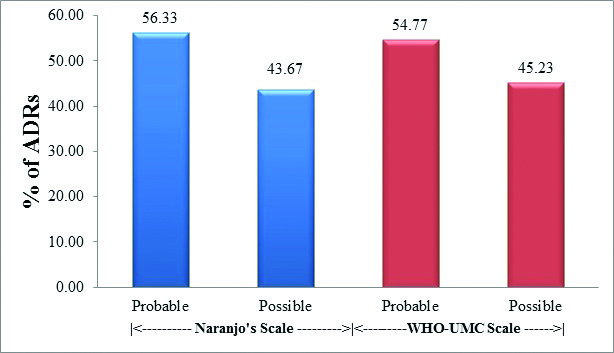
Causality assessment by both Naranjo’s algorithm and WHO– UMC scale classified majority of ADRs as ‘probable’ followed by ‘possible’. There were no cases of ‘definite’ or ‘certain’ ADR on Naranjo’s as well as WHO – UMC scale, respectively [Table/Fig-7]. The association of results with both the scales was highly significant. (Fisher’s-exact test – p < 0.001).
Causality assessment of ADRs using Naranjo’s algorithm and WHO-UMC scale. (n = 703).
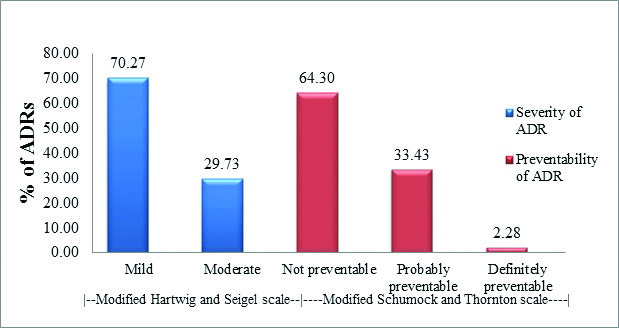
Majority of ADRs were assessed as mild (n=494) according to modified Hartwig and Seigel scale. There were no cases of severe or fatal, life-threatening ADRs. Most of the ADRs were assessed to be not preventable (n=452) according to modified Schumock and Thornton scale [Table/Fig-8]. The association between gender and severity or preventability of ADR was not statistically significant. (Chi-Square test – p>0.05).
Severity (Modified Hartwig and Seigel scale) and Preventability (Modified Schumock and Thornton scale) assessment (n = 703).
Discussion
The spectrum of pharmacovigilance is rapidly expanding in our country. Globally, pharmacovigilance data is usually available for individual drugs or drug groups; whereas, there is scarcity of data for ADR profiles in specific disorders. Bipolar disorder is a common, recurrent and frequently debilitating psychiatric disorder [4]. The drugs used in the management of bipolar disorder have significant adverse effects which decrease patient compliance and increase cost of therapy. Our study has reported the pattern of ADRs in bipolar disorder patients who were being treated with one or more medications.
In this study, majority of patients were diagnosed with bipolar disorder type I (92.78%) which corresponds with the higher incidence of bipolar disorder type I worldwide [12]. Among the patients who developed ADRs, the percentage of male patients was slightly more as compared to female patients. This is similar to the study by Sengupta G et al., [13].
The most common age group in which ADRs were observed was in the 31-40 years group (33.33%). The study done by Sengupta G et al., have quoted mean age of patients with ADRs within the range observed in our study [13].
Thirty different types of ADRs were noted in our study. The most common being asthenia followed by sedation, polyuria/polydipsia, weight gain, tremors and Extrapyramidal Side Effects (EPS). This may be due to an extension of the pharmacological actions of the drugs used in the treatment of bipolar disorder.
Asthenia was the most common ADR observed in our study. It was mild in all the cases and did not require any change in the medication or reduction in its dose. We could not find any studies quoting asthenia as a common adverse reaction. This may be due to lack of awareness regarding availability of specific scales for registration of adverse reactions due to psychotropic drugs such as UKU side effects rating scale, which we have applied in our study. Sedation was seen with the use of atypical antipsychotics and benzodiazepines. In most cases, it was mild and did not interfere with the routine activities of the patient. In nearly 4% of the cases where it impaired the motor or cognitive performance of the patient, the dose of the medication was decreased or a switch over to a non-sedating antipsychotic was made. Extra pyramidal side effects were seen with the use of antipsychotics.
EPS was more commonly associated with the use of risperidone. In all cases of EPS the reaction was treated by giving trihexiphenidyl and in eight cases, the drug was changed or dose was decreased. Olanzapine was responsible for more than half of the cases of weight gain. Diet control with exercise was advised in majority; however, in two cases topiramate was prescribed to treat weight gain. Tremors were seen throughout observation period, but the maximum incidence was observed during the initial phase of treatment. Tremors were generally mild in most of the patients, which resolved on reducing the dose. In 18 cases of moderate to severe tremors, propranolol was prescribed for treatment. Most patients receiving lithium had polydipsia and polyuria reflecting mild benign Nephrogenic Diabetes Insipidus (NDI). In four cases of lithium-induced renal dysfunction, carbamazepine was prescribed. A study conducted by De Bragança AC et al., demonstrated the efficacy of carbamazepine in lithium-induced NDI [14].
In our study, hypothyroidism was more common in women than in men, which correlates with the findings of Ahmadi-Abhari SA et al., [15]. In all cases of lithium–induced hypothyroidism, lithium was stopped and another mood stabilizer was substituted (most commonly valproate). The most common sexual side effect seen was decreased libido. Sexual dysfunctions such as ejaculatory dysfunction and erectile dysfunction were seen in male patients receiving antidepressants. Fluoxetine was the most commonly responsible selective serotonin reuptake inhibitor for these adverse effects. In a recent randomized controlled trial done by Khazaie H et al., in patients of major depressive disorder; fluoxetine was associated with most cases of sexual dysfunction in both males and females [16]. In patients with GI disturbances, anaemia, acne and alopecia either the drug was stopped or the dose was decreased. No other ADR required any stoppage or change of drug.
Mood stabilizers were associated with majority of ADRs. Among them, lithium was found to be responsible for 35.56% of total ADRs, making it the commonest single drug implicated followed by valproate. This may be partly due to higher rates of prescription for lithium as a first line drug for acute mania as well as maintenance phases of bipolar disorder. Atypical antipsychotics were also frequently prescribed, most common drug associated with ADRs being olanzapine followed by risperidone, which is similar to the findings of Lucca JM et al., [17].
Regarding causality assessment, maximum cases were classified as ‘probable’ according to both Naranjo and WHO-UMC causality assessment scales. Our study had no ‘certain’ ADR on WHO-UMC scale since re-challenge was not performed by clinician once the drug was withdrawn. This is similar to other studies performed in this setting [13,18].
Most of the reactions were of mild to moderate severity. The reactions which were mild level 1 or 2 were asthenia, accommodation disturbances, increased salivation, GI disturbances and dermatological reactions. ADRs such as EPS, tremors, sexual dysfunctions, and hypothyroidism were assessed as ‘moderate level 3 or 4’. There were no cases of severe level 5, 6 or 7 ADR in our study.
In the preventability assessment, ~ 35% ADRs were found to be preventable. Lithium accounted for 12% of preventable ADRs which is similar to the findings of Luppa CA et al., [19]. While most of the ADRs were ‘not preventable’ some of the reactions like vomiting and constipation were ‘definitely preventable’ and the others such as diarrhoea, EPS and weight gain were ‘probably preventable’.
Limitation
There were chances of missing certain ADRs during the study period since some of them may have been transient or not severe enough to significantly trouble the patient to remember it and report. Routine haematological and biochemistry reports were usually available, but ECG screening of the patients or taking regular blood samples for thyroid function tests, prolactin measurement and therapeutic drug monitoring was not possible. The study was done in OPD patients only and thus indoor patients of bipolar disorder were excluded. Also, for logistical reasons, we interviewed the patients only during morning OPD timings from 9.30 am to 12.30 pm. So, it is possible that we may have missed some bipolar disorder patients who came during the evening OPD.
Conclusion
This study is one of its kinds as it details the pattern of ADRs occurring in patients of bipolar disorder. It illustrates the ADRs likely to be encountered in patients with bipolar disorder being treated with psychotropic drugs in the setting of an Indian tertiary care hospital. From this study, we can conclude that ADRs occur quite frequently in these patients which are often mild in nature and are more commonly associated with the mood stabilizers, particularly lithium. Thus, the recognition of these side-effects and their management can ensure optimal care for the patient. Such prospective studies conducted across multiple hospitals through active collaboration of psychiatrists and pharmacologists can be helpful in building up a database for ADRs occurring due to psychotropic drugs. It emphasizes the need to develop an attitude for pharmacovigilance among the treating physicians so that we could precisely monitor the ADRs caused by all the drugs used in the management of bipolar disorder. Availability of complete pharmacovigilance data including the follow up details of the patients will go a long way in making the analysis adequate and accurate.