Clinical strains of micro-organisms are collected directly from the oral cavity and cultured whereas standard strains of micro-organims are obtained in specific atmospheric pressure and specific temperature in laboratory chambers. Several disinfectant [13-15] have been suggested for disinfection of prosthesis but research data on clinical strains is limited [13,14]. The study focuses on the comparative evaluation of effectiveness of four commercially available disinfectants on heat cure acrylic resin samples contaminated with standard and clinical strains of two micro-organisms (Candida albicans and Streptococcus mutans) commonly inhabiting the oral microflora.
Materials and Methods
The in vitro experimental study was conducted at DA Pandu Memorial Rashtreeya Vidyalaya (DAPMRV) Dental College, Bangalore, India. A total of two hundred resin samples were fabricated. Out of which, one hundred were utilised for standard strains and one hundred for clinical strains of micro-organisms [Table/Fig-1]. From the hundred samples for standard strains, fifty were utilised for Candida albicans and other fifty for Streptococcusmutans. Similarly, from the hundred samples for clinical strains, fifty were utilised for Candida albicans and other fifty for Streptococcusmutans. The control groups (G1 and G2) that were contaminated separately with the two micro-organisms were not subjected to any disinfectant.
Grouping and number of CFU formed and intergroup comparison for standard and clinical strains of micro-organisms treated with disinfectants.
| Disinfectants (D) | STANDARD STRAINS OF MICRO-ORGANISMS (Selective culture media is not essential) |
|---|
| Candida albicans(C) ATCC 10231 | Streptococcus mutans(S) ATCC 35688 |
|---|
| Group (No. of samples) | Mean (CFU/ml) | Group (No. of samples) | Mean (CFU/ml) |
|---|
| Control group [G1] | G1-C (10) | >105 | G1-S (10) | >105 |
| D1-1% Sodium hypochlorite | D1-C (10) | <10,000 | D1-S (10) | <10,000 |
| D2-2% Chlorhexidine Digluconate | D2-C (10) | 10,000 | D2-S (10) | >10,000<25,000 |
| D3 -2% Glutaraldehyde | D3-C (10) | >5000<10,000 | D3-S (10) | 25,000 |
| D4-3.8% Sodium Perborate | D4-C (10) | 25,000 | D4-S (10) | >25,000<50,000 |
| p-value (ANOVA) | p<0.001(HS) | p<0.001(HS) |
| CLINICAL STRAINS OF MICRO-ORGANISMS |
| Disinfectants (D) | Candida albicans(C) | Streptococcus mutans(S) |
| Group (No. of samples) | Selective culture media (incubation period in hrs) | Mean (CFU/ml) | Group (No. of samples) | Selective culture media (incubation period in hrs) | Mean (CFU/ml) |
| Control group [G2] | G2-C (10) not subjected to disinfection | >105 | G2-S (10) not subjected to disinfection | >105 |
| D5-1%Sodium hypochlorite | D5-C (10) | Sabouraud’s Dextrose Agar (72) | <10,000 | D5-S (10) | Tryptic Soy Agar (24) | 10,000 |
| D6-2% Chlorhexidine Digluconate | D6-C (10) | Sabouraud’s Dextrose Agar (72) | 25,000 | D6-S (10) | Tryptic Soy Agar (24) | 25,000 |
| D7 -2% Glutaraldehyde | D7-C (10) | Sabouraud’s Dextrose Agar (72) | 10,000 | D7-S (10) | Tryptic Soy Agar (24) | >10,000 <25,000 |
| D8-3.8% Sodium per borate | D8-C (10) | Sabouraud’s Dextrose Agar (72) | 50,000 | D8-S (10) | Tryptic Soy Agar (24) | 50,000 |
| p-value (ANOVA) | p<0.001(HS) | p<0.001(HS) |
The randomization of sampling was done by selecting any five samples (out of ten) and obtaining data from them (termed as observations).
The methodology included: I) Sample fabrication; II) Collection of micro-organisms; and III) Evaluation of antimicrobial activity of disinfectants.
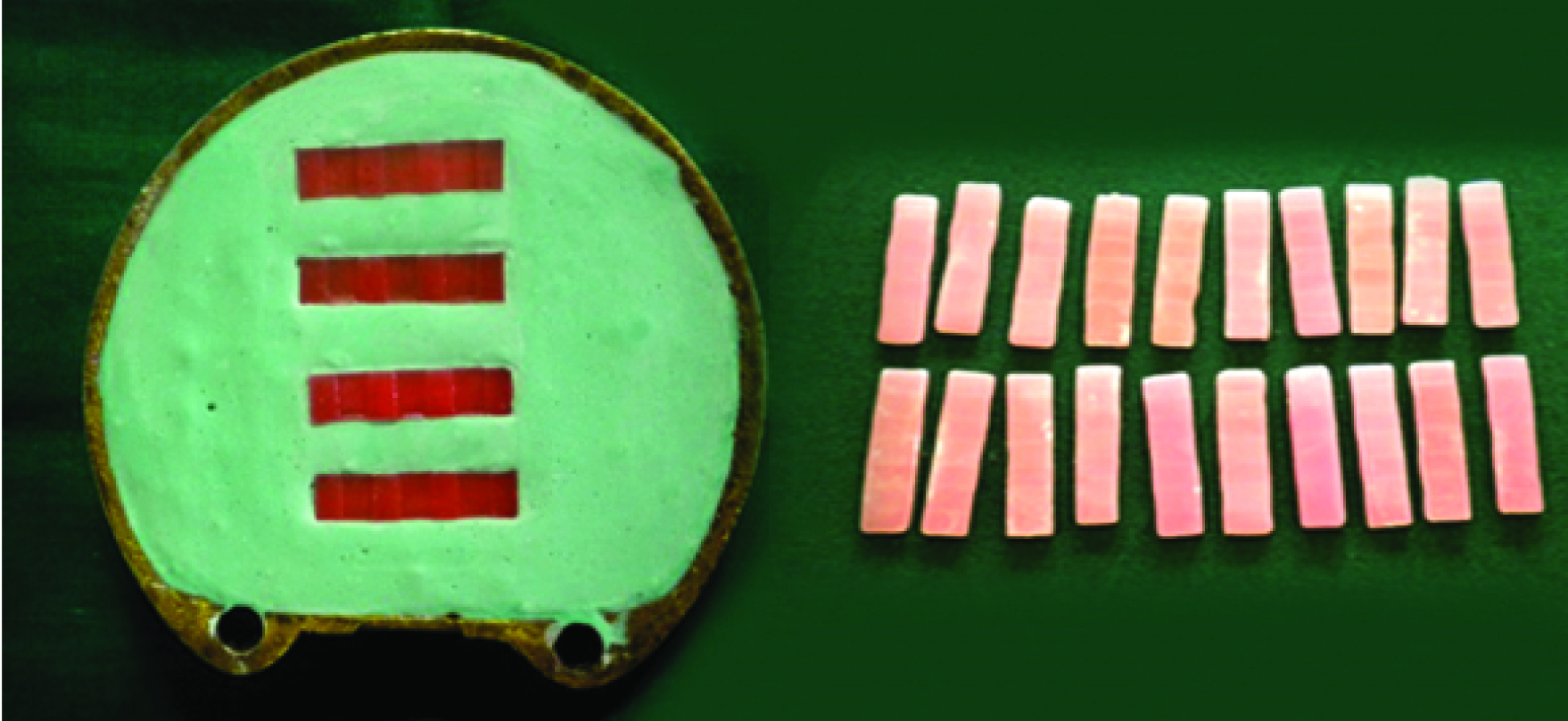
I) Sample Fabrication: A stainless steel mould measuring 30x7 x2 mm3 (ISO 1567) was fabricated. Molten wax was poured into it. Lower member of the flask was filled with freshly mixed dental stone (Type III, Kalastone; Kalabhai Pvt. Ltd. Mumbai, India) and four wax samples were placed into it. Dental stone was contoured such that the samples were in level with the investing medium. Care was taken to ensure sufficient space between the investing medium and each sample analogues. A thin coat of petroleum jelly was coated onto the investing medium. The body of flask was positioned on top of the base. A second mix of dental stone was prepared with recommended water/powder ratio and poured. Care was taken to ensure that the investing medium achieves intimate contact with all the surfaces. After the final set, dewaxing was done. Heat cure acrylic resin (DPI Heat Cure; Dental Products of India. Ltd,) was mixed according to manufacturer’s instructions and was packed. Curing was done by using electrically controlled polymerization unit (KaVo). The curing temperature was 70°C for eight hours with no terminal boiling treatment. The cured samples were retrieved; trimmed and standard methods of finishing and polishing were performed and stored in distilled water until the experiment [Table/Fig-2].
Fabrication of the acrylic resin samples
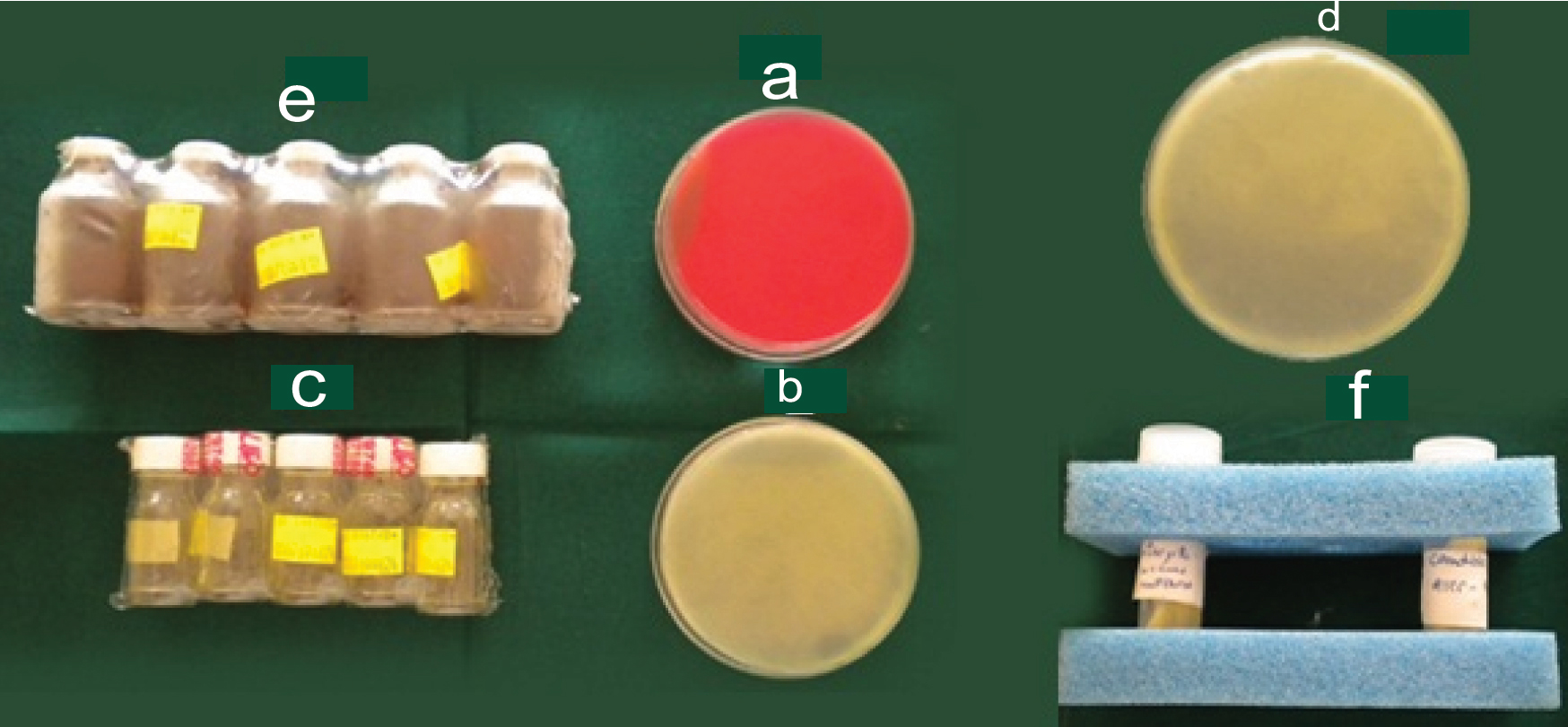
II) Collection of micro-organisms: For standard strains, confirmation of organism was not required since particular microbial strain was cultured in standardised conditions in the microbial laboratory. The Department of Microbiology, DAPMRV Dental College and Hospital procured the standard strains from The Institute of Microbiology Technology, Chandigarh, India. For clinical strains, a sterile cotton swab was passed over the buccal mucosa of a healthy patient (55-year-old) without any systemic illness. The culture media required for growth of micro-organisms are shown in [Table/Fig-3], a) Blood agar used for identification of micro-organisms; b and c) Sabouraud dextrose agar and broth used as selective culture media for Candidaalbicans; d and e) Tryptic Soy Agar and broth used as selective culture media for Streptococcus mutans; F) Standard strains of Streptococcus mutans and Candida Albicans (ATCC 35688 and ATCC 10231).
Culture media for growth of micro-organisms. a) Blood Agar used for identification of micro-organisms; b,c) Sabouraud’s Dextrose Agar and broth used as selective culture media for Candida albicans; d,e) Tryptic Soy Agar and broth used as selective culture media for Streptococcus mutans; and f) Standard strains of Streptococcus mutans and Candida Albicans (ATCC 35688 and ATCC 10231).
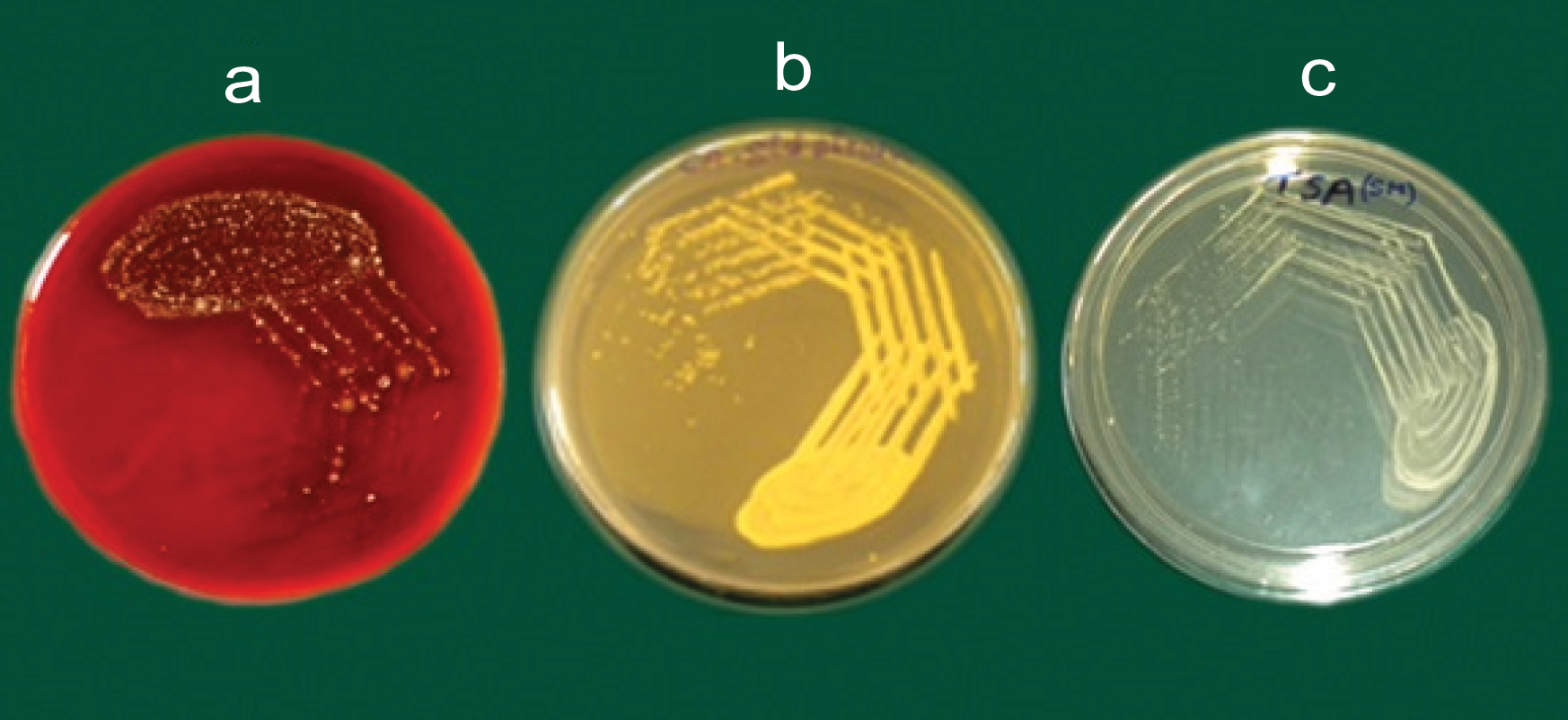
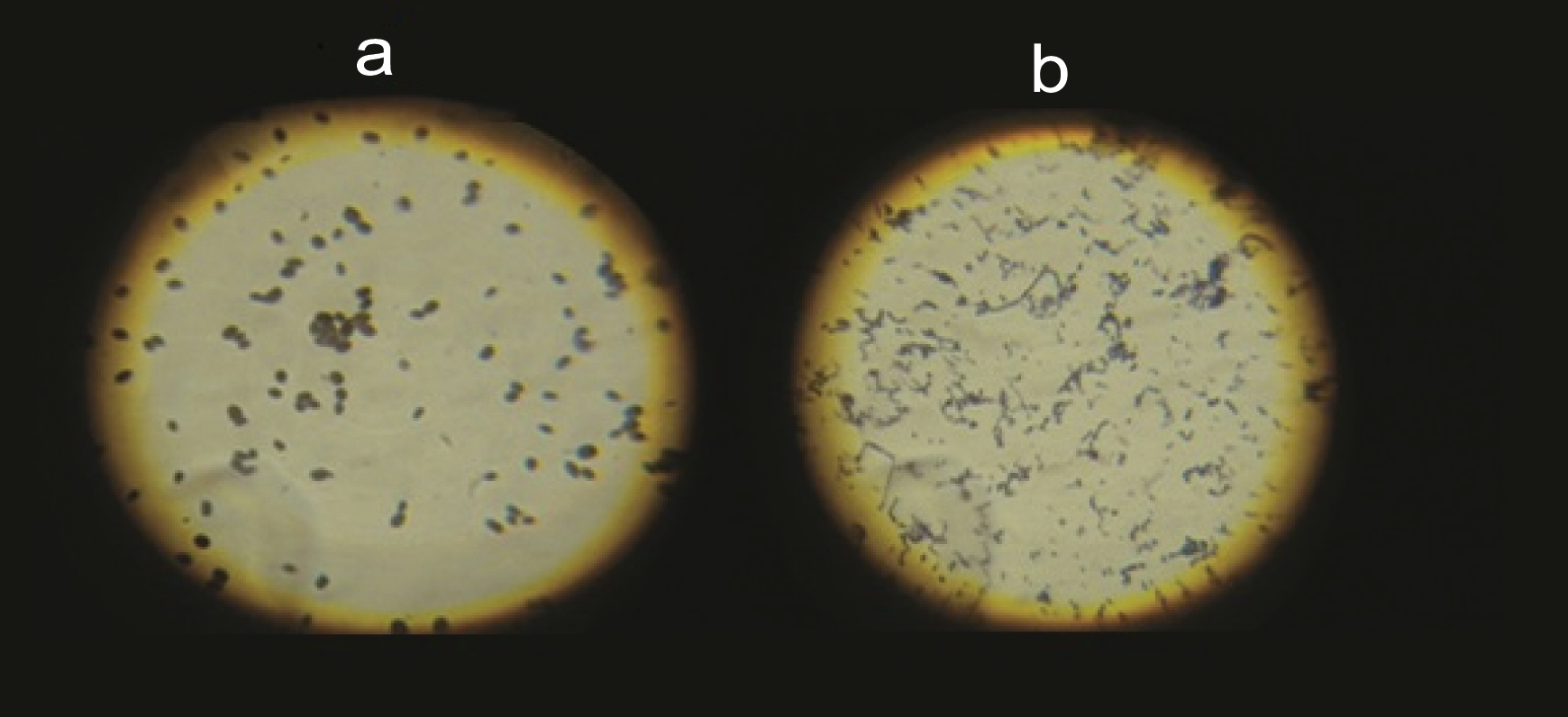
The oral swab was cultured on Blood agar plate [Table/Fig-4], incubated at 37°C for 24 hours and growth of the microbial colonies was observed after 24 hours. A thin smear was prepared, Gram staining (for the identification of the micro-organisms) was done and organisms were observed microscopically. Candida albicans were observed as Gram-positive oval budding yeast like cells and Streptococcus mutans as Gram-positive cocci in long and short chains [Table/Fig-5].
a) Culture of oral swab on blood agar plate; Standard strain of b) Candida Albicans; and c) Streptococcus mutans.
Microscopic view of a) Candida albicans; and b) Streptococcus mutans.
Following the organism identification, confirmation was done by growing them in selective culture media [Table/Fig-1,4]. The parameters of optical density corresponding to suspensions of 1x106 CFU of each micro-organism were spectrophotometrically observed in sterile saline solution. Each sterile resin specimen was then transferred to a tube containing tryptic soy broth and sabouraud dextrose broth respectively and inoculated with 0.1 ml of the standardized suspension of each micro-organism [Table/Fig-6]. Tubes were incubated for 24 hours at 37°C for Streptococcusmutans and 72 hours for Candida albicans [Table/Fig-1,6]. After the appropriate incubation period for particular micro-organism, 0.1 ml of this suspension was plated on selective culture media and numbers of CFUs were counted. This indicated CFU before disinfection.
Incubation of tubes containing Tryptic Soy Broth (TSB) and Sabouraud’s Dextrose Broth (SDB), inoculated with 0.1 ml of the standardized suspension of each micro-organism.
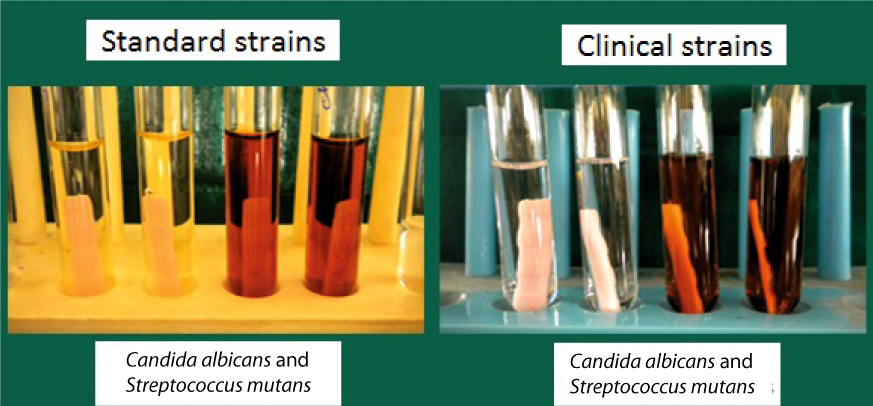
III) Evaluation of antimicrobial activity of disinfectants: After incubation, each specimen was immersed in a tube containing 10 ml of disinfectant to be tested [Table/Fig-1]. After removing from the disinfectant, the samples were immersed for two seconds in sterile distilled water to eliminate excess of disinfectant. They were then transferred to tubes containing 10 ml of sterile saline solution (Sodium Chloride 0.85%) and adhered cells were dispersed. Almost 0.1 ml of this suspension was again plated on tryptic soy and sabouraud dextrose agar respectively. Plates were incubated and CFU were counted. This indicated CFU after disinfection. The control groups were not subjected to any disinfection procedure [Table/Fig-1].
The data obtained were subjected to ANOVA and Tukey’s test and the significance level were interpreted as p<0.001 as Highly Significant (HS), p≤0.05 as Significant (S) and p>0.05 as Non-Significant (NS).
Of the 10 samples in each group, five were selected at random and the number of CFU/ml were measured. Each of these measurements was termed as observation. So for each case, the observations made at random were called first, second, third, fourth and fifth values for accuracy of measurement and the mean was obtained. The intergroup and intragroup values were subjected to ANOVA and Tukey’s test.
Results
Control groups (G1 and G2) were not subjected to any disinfection and as expected, they showed maximum mean CFU/ml (>105). Standard strains of Candida albicans (C) showed varied mean CFU/ml from <10,000 (D1-C;1% sodium hypochlorite) to 25,000 (D4-C; 3.8% sodium perborate). Similarly standard strains of Streptoccocus mutans (S) showed variation of mean CFU/ml from <10,000 (D1-S; 1% sodium hypochlorite) to <50,000 (D4-S; 3.8% Sodium perborate).
Clinical strains of Candida albicans (C) showed varied mean CFU/ ml from <10,000 (D5-C; 1% sodium hypochlorite) to 50,000 (D8-C; 3.8% sodium perborate) and clinical strains of Streptoccocusmutans (S) showed variation of mean CFU/ml from 10,000 (D5-S; 1% sodium hypochlorite) to 50,000 (D5-S; 3.8% sodium perborate).
Statistical analysis showed intergroup comparisons as highly significant (HS) with p<0.001 [Table/Fig-1] and intragroup comparisons as significant (S) with p<0.05 with the exception of comparison between clinical strains of Streptococcus mutans subjected to 2% chlorhexidine digluconate (D6-S) and clinical strains of Streptococcus mutans subjected to 2% glutaraldehyde (D7-S), which was found to be NS with p>0.05 [Table/Fig-7,8].
Intra-group comparisons (Tukey’s test) for standard strains of both micro- organisms.
| Candida albicans | Streptococcus mutans |
|---|
| D1-C | D2-C | D3-C | D4-C | | D1-C | D2-C | D3-C | D4-C |
|---|
| G1-C | p<0.05 (S) | p<0.05 (S) | p<0.05 (S) | p<0.05 (S) | G1-S | p<0.05 (S) | p<0.05 (S) | p<0.05 (S) | p<0.05 (S) |
| D1-C | - | p<0.05 (S) | p<0.05 (S) | p<0.05 (S) | D1-S | - | p<0.05 (S) | p<0.05 (S) | p<0.05 (S) |
| D2-C | - | - | p<0.05 (S) | p<0.05 (S) | D2-S | - | - | p<0.05 (S) | p<0.05 (S) |
| D3-C | - | - | - | p<0.05 (S) | D3-S | - | - | - | p<0.05 (S) |
Intra-group comparisons (Tukey’s test) for clinical strains of both micro-organisms.
| Candida albicans | Streptococcus mutans |
|---|
| D6-C | D7-C | D8-C | | D6-S | D7-S | D8-S |
|---|
| D5-C | p<0.05 (S) | p<0.05 (S) | p<0.05 (S) | D5-S | p<0.05 (S) | p<0.05 (S) | p<0.05 (S) |
| D6-C | - | p<0.05 (S) | p<0.05 (S) | D6-S | - | p>0.05 (NS) | p<0.05 (S) |
| D7-C | - | - | p<0.05 (S) | D7-S | - | - | p<0.05 (S) |
Discussion
Contact of dental prosthesis with oral tissues, saliva and blood creates a medium for the transfer of organisms from patients to dental and laboratory personnel. Powell GL et al., surveyed dental laboratories and found that 67% of all materials sent from dental offices to dental laboratories were contaminated with varying degrees of opportunistic pathogenicity (mainly Streptococcus species, Candida species, Escherichia coli, Pseudomonas species) [13]. This spread of infection can be largely reduced by disinfecting the prosthesis before sending to and also after receiving from laboratory [14].
Various studies were conducted to evaluate efficiency of sodium hypochlorite on resins. These include 5.25% sodium hypochlorite solution proved effective against Staphylococcus aureus, Candidaalbicans and Escherichia coli within four minutes [15], 1% sodium hypochlorite effective against micro-organisms contaminated by removable prosthesis [16] and on resilient lining materials [17]. However, denture soaking in 5.25% sodium hypochlorite has shown to have deleterious effects on denture materials. It exhibits a bleaching effect on resin; tarnish and corrosion of cobalt chromium alloys [18]. According to Molinari JA and Runnells RR, The Center for Disease Control has recommended the use of 0.05%-0.5% sodium hypochlorite as an effective agent in infection control protocol [19]. It is proved to be more effective than exposure to microwave energy in disinfection of longterm soft lining material [20]. Hypochlorite denture cleansers are fungicidal and are known to be effective by dissolving mucin and other organic substances. The method of sterilization by immersing the dentures leaves the resin surface relatively unchanged and therefore possibly less susceptible to plaque accumulation.
Evidence has shown that mechanical cleaning alone (i.e., brushing) is not sufficient to remove denture plaque and chemical cleaning (i.e., immersion or brushing with disinfectants) is essential. These include dentifrices, proprietary denture cleansers, mild detergents, household cleansers, bleaches and vinegar. Both immersion and brushing techniques were widely used with these materials [20-22]. The most common commercially available denture cleansers use immersion techniques (marketed in powder and tablets forms).
Immersion agents contain alkaline compounds, detergents, sodium perborate and flavoring agents. When dissolved in water, sodium per-borate decomposes to form alkaline peroxide. This peroxide subsequently releases oxygen, which is reported to loosen debris via mechanical means [4,23,24].
Toothbrushes in conjunction with most dentifrices, mild detergents and soaps do not appear to be harmful. Conversely, household cleansers such as kitchen and bathroom abrasives are definitely contraindicated. Prolonged use of such cleansers may significantly alter the function and aesthetics of resin prosthesis. As a result, every patient should be educated regarding the care and cleansing of resin prosthesis [22].
Hence, few of the most commonly used disinfectants such as sodium hypochlorite; glutaraldehyde, chlorhexidine and sodium perborate [3,15-17,25-29] were used in present study. The micro-organisms namely Streptococcus mutans and Candidaalbicans were studied since they are common commensals of oral cavity. The selection of micro-organisms was based on the pathogenic potential or representative importance for antimicrobial effectiveness evaluation studies [5,6,8-10]. Streptococcus mutans is part of the normal oral microflora. Its presence out of this oral cavity may be used as a contamination indicator [3]. Candida albicans (due to traumatising prosthesis, unsatisfactory oral hygiene conditions and systemic factors) is associated with denture stomatitis; a very common problem associated with denture wearers [3,8-12]. Therefore, literature focuses on this micro-organism not only as a cross infection problem, but also as a denture stomatitis related factor. The study was conducted on standard and clinical strains since the effectiveness of disinfectants on both strains is different due the difference in the virulence of standard and clinical strains [26].
There are many denture cleansers marketed for patients, most of them contain sodium perborate in varying concentration, available as powder and tablet forms. Studies done on various disinfectants, have shown that sodium perborate can be substituted with more effective disinfectants like sodium hypochlorite [3,15-17,25-29]. The results of the present study when correlated with the previous studies, showed similar results-“sodium hypochlorite, the most effective disinfectant”
Even though, sodium hypochlorite has proved to be the most effective disinfectant indicated from various studies by different researchers [3,15-17,25-29], it is not popularly prescribed. Moreover, still extensive research and clinical trials comparing only sodium hypochlorite and sodium perborate are required to prove sodium hypochlorite as the most popular and commonly used disinfectant among denture wearing patients, thus rendering the near to best disinfectant so that prosthesis sterilization is performed even out of the dental office by the patient.
Disinfection procedure commences not only prior to attending a patient but to be continued during treatment and post-treatment. It is an act, well studied by the operator and taught to the patient for the betterment.
Limitation
The limitations of the study were: (1) Growth of different species of micro-organisms was inevitable; (2) Culturing a particular strain of micro-organism was a sophisticated procedure; (3) Contamination of culture plates which could be due to accidental contact of some equipment with the culture media, cross contamination.
Conclusion
Within the limitations of the study, it was concluded that the most effective disinfectants for standard and clinical strains of Candidaalbicans and clinical strains of Streptococcus mutans is 1% sodium hypochlorite followed by 2% glutaraldehyde and 2% chlorhexidine digluconate. The most effective disinfectants for standard strains of Streptococcus mutans is 1% sodium hypochlorite followed 2% chlorhexidine digluconate and 2% glutaraldehyde. The least effective disinfectant for standard and clinical strains of the two micro-organisms is 3.8% sodium perborate.