Atherosclerosis is a multifactorial disease of arteries and a cause of many cardiovascular complications. It is generally characterized by increased size, endothelial dysfunction and vascular inflammation [1]. It commonly coexists with pro-atherogenic disorders. Considerable evidence is available to support the association between vascular disease and steroid induced insulin resistance [2]. By looking into clinical histories and autopsies, few researchers identified the association between obesity, hypertension, abnormal glucose metabolism and existence of atherosclerosis [3]. The lipoprotein lipase levels are also reduced with insulin resistance and results in decrease of VLDL clearance, which may make a smaller contribution to elevated TGs in this setting. Further, VLDL is metabolized to remnant lipoprotein and LDL, increased concentration of triglyceride rich VLDL particles contribute to abnormal HDL metabolism in insulin resistance condition and are strongly associated with atherosclerotic risk [4]. The cell organelle may undergo stress due to nutrients excess and that results in the development of atherosclerosis and insulin resistance. Disruption of homeostasis may occur at cell organelle that may result into ER and genomic stress due to excess glucose delivery. The factors affecting nuclear genome are oxidative stress and also due to accumulation of lipids inside the cells. They disrupt protective mechanisms that minimize the damage due to inflammation [5].
Glucocorticoids (GCs) are the key hormones that regulate glucose homeostasis and it is released in response to stress and low blood-glucose concentration. Excess of GC causes insulin resistance [6] and prolonged corticosteroid therapy accelerates the development of atherosclerosis [7]. Dexamethasone is a potent glucocorticoid, has high affinity for glucocorticoid receptors with minimal mineralocorticoid property. Several authors tried variable doses of dexamethasone to induce insulin resistance in rodents [8,9]. Dexamethasone treatment can also induce dose dependent changes in aorta as moderate and severe thickening of tunica media was observed in respective doses of 4 and 8 mg/kg dexamethasone [10].
The current study is part of the previously conducted study in which the preventive effects of CZE were evaluated against dexamethasone induced insulin resistance [11], and now it is intended to evaluate the anti-atherosclerotic potential of aqueous extract of C. zeylanicum bark against dexamethasone induced dyslipidemia and atherosclerosis in rats.
Materials and Methods
This experimental study was carried out at Department of Pharmacology, KS Hegde Medical Academy, Mangalore, Karnataka, India, from August 2013 to January 2014.
Experimental Animals
A sum of 36 healthy male Wistar albino rats, weighing around 230-270 gm were chosen for this study. The animals were housed at 22-24°C temperature and maintained under 12 hour light -12 hour dark cycle for two weeks ad libitum. An approval from IAEC was obtained (Letter no: AEC/29//2011) prior to the study. All the procedures in this study were performed as per the CPCSEA revised guidelines.
Grouping
Animals were equally allocated into five treatment groups and one plain control with six animals in each [Table/Fig-1].
Grouping of animals (n=6).
| Sl. No | Groups |
|---|
| I | Plain control (PC) |
| II | Dexa control (DC) |
| III | Dexa+ ROSI 8 mg/kg |
| IV | Dexa+ ROSI 16 mg/kg |
| V | Dexa+ CZE 250 mg/kg |
| VI | Dexa+ CZE 500 mg/kg |
Note: Dexa- Dexamethasone, ROSI-Rosiglitazone, CZE- C.zeylanicum bark extract
Plant Material and Extraction
Cinnamon (C.zeylanicum) bark was procured from SDM Ayurvedic pharmacy, Udupi, Karnataka in Dec 2013. The bark was dried, grinded, finely powdered and then subjected to multiple cycles of extraction in Soxhlet apparatus using distilled water at 80°C temperature. In each cycle, 50 gm of bark powder was packed and placed inside the Soxhlet chamber. The yield was concentrated by using rotary evaporator at reduced pressure [14]. A final yield of 6.1% extract was collected and stored in desiccator and used during this study [11].
Drugs and Doses
The doses were selected based on a pilot study that was conducted in a small group of animals.
Aqueous extract solution of C. zeylanicum (CZE) was prepared as 250 mg/ml and 500 mg/ml concentrations with distilled water. Group V and VI received 250 mg/kg and 500 mg/kg respectively. Rosiglitazone was procured from Sigma labs in a dry powdered form and it was suspended in 2% gum acacia solution. Nearly, 8 mg/kg and 16 mg/kg doses were advised orally to group III and IV respectively. However, dexamethasone injection 8 mg/kg body weight/day (intraperitoneal injection) was used in respective groups [11].
Study Procedure
The above mentioned doses of CZE and ROSI were administered everyday throughout the study period (12 days). Dexamethasone is long acting and highly potent steroid over others and devoid of mineralocorticoid activity [15,16]. However, dexamethasone treatment was initiated on day 7 and continued till last day of the study period. Each rat in each group was allowed to have 100gm of standard food pellets and 100 ml of water. On day 11, all the rats were fasted overnight and accessed water alone. The very next morning, respective drugs were administered to all groups two hours before collecting blood samples. All the blood samples were collected by puncturing retro orbital plexus under either anaesthesia and were centrifuged at 4000 rpm/20 min. The serum was separated from the sediment and processed for the estimation of lipid profile.
Estimation of Serum Lipids
The lipid profile was determined by using standard laboratory reagents and automatic analyser. The HDL cholesterol was determined by phosphotungstate magnesium acetate precipitation method, TGs were determined by GPO-PAP method and the serum total CH was determined by oxidase peroxidase method [17].
The VLDL and LDL were determined by using standard formulae which follows as:
VLDL= Triglycerides/5 and LDL= Total cholesterol – (HDL cholesterol – Triglycerides/5) respectively.
Atherogenic Index
The Atherogenic index is used to understand and assess the risk of development of atherogenicity in vessels. As the AI value rises the risk of atherogenesis also increases. It was employed and calculated in respective study groups by using the following formula [17].
• Atherogenic index (AI) = Total CH- HDLC/HDLC
Isolation of orta for the Histopathological Examination
Following the collection of fasting blood samples, rats were sacrificed by cervical dislocation. On ventral position the chest of the animal was opened with incision, aorta was traced and a distance of 2 cm was dissected away. Isolated tissues were fixed in 10% formalin and were employed for further histopathological observations. Sections were made 5 μm thick and stained with Haematoxylin and Eosin (H&E stain) and were observed for atherogenic/atherosclerotic changes in the aorta [18].
Measurement of Thickness of Aorta
The aortic sections were studied under 400X under Olympus photographic microscope and photomicrographs were captured. The thickness of the aorta in the photomicrographs was measured with the help of Image J 1.41o version [18].
Percent (%) difference in the thickness of aorta was calculated among study groups compared to PC by using following formula.

Note: PC-Plain control; SG- Study group (DC, CZE, ROSI)
Percent (%) difference in the thickness of aorta was calculated in treatment groups compared to DC by using following formula.

Note: DC-dexa control; TG- treatment group (CZE, ROSI)
Statistical Analysis
Suitable data was represented as Mean±S.D. The statistical analyses were performed using One-way ANOVA followed by Dunnet post-Hoc test. Statistical significance was assumed at the 5% level of significance and p<0.05 was considered as significant.
Results
Effect of CZE and ROSI on Serum lipids
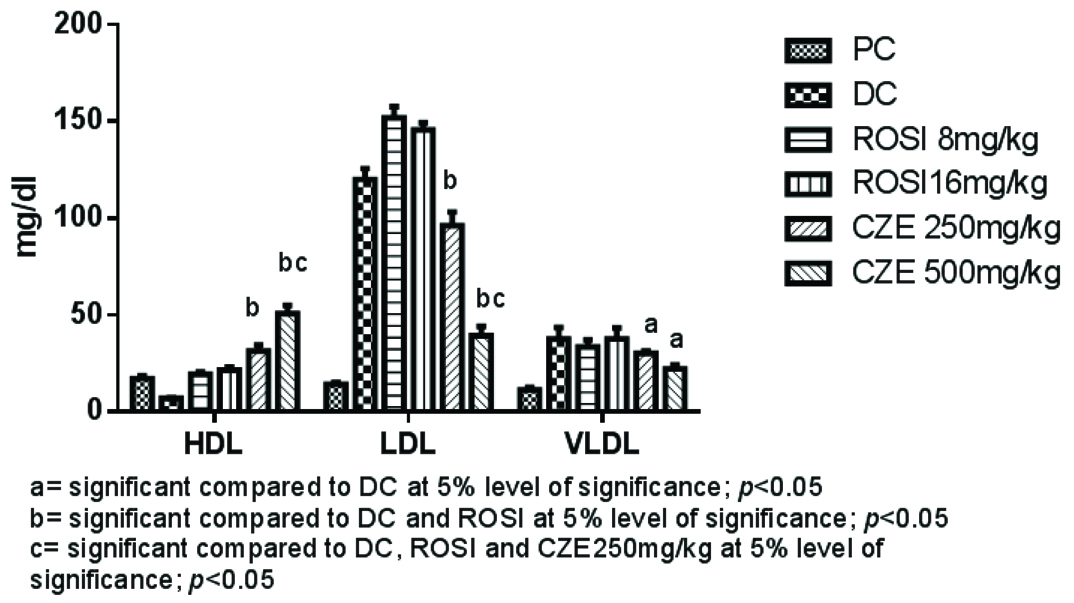
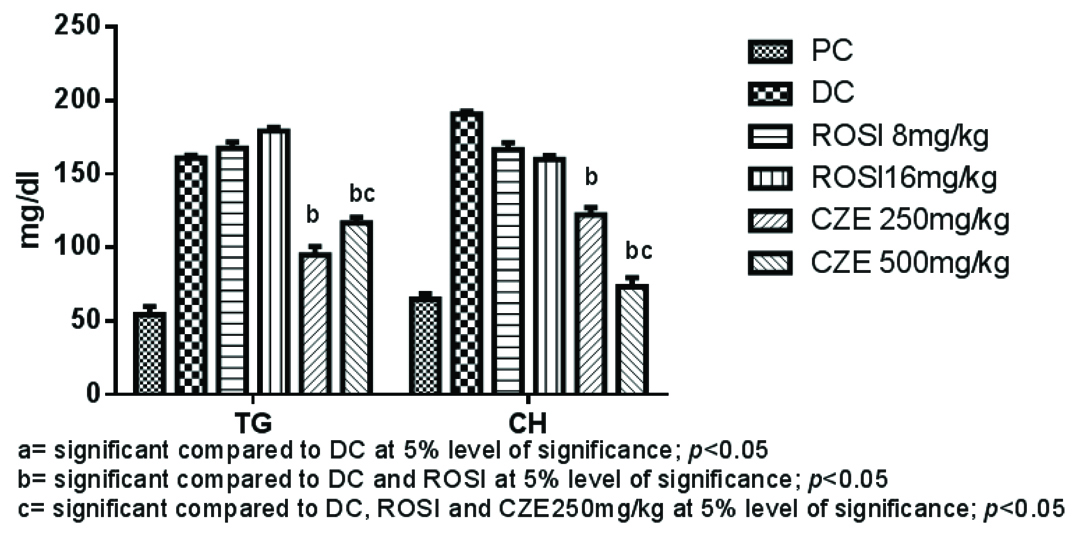
In present study, treatment with high dose of dexamethasone (8 mg/kg i.p) caused significant fall in High Density Lipoproteins (HDL) levels and significantly raised TG, total CH, LDL and VLDL (p<0.05). Whereas, CZE 500 mg/kg treatment significantly elevated HDL levels compared to DC and both 8 mg/kg and 16 mg/kg of ROSI treated groups. CZE 500 mg/kg caused marked fall in LDL, VLDL, TG and CH levels compared to DC and both the ROSI treatments (p<0.05). However, there was no significant difference observed between CZE 250 mg/kg and 500 mg/kg treatments in reducing VLDL alone (p>0.05). Both the ROSI treatments (8 mg/kg and 16 mg/kg) improved serum HDL markedly compared to DC (p<0.05). Although, there was no significant difference between both ROSI treatments in reducing LDL, VLDL, TG and CH and raised HDL levels (p>0.05) [Table/Fig-2,3].
Serum HDL, LDL and VLDL levels in plain control and treatment groups.
Serum TG and CH levels in plain control and treatment groups.
Effect of CZE and ROSI on Atherogenic index (AI)
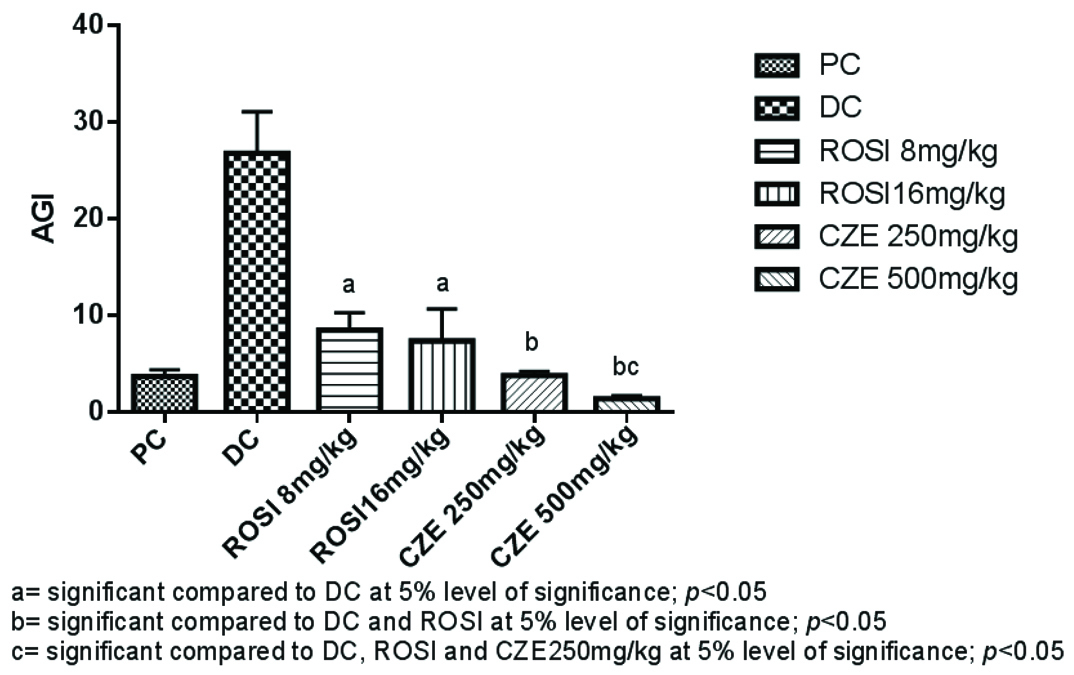
Dexamethasone significantly raised the AI compared to PC group and CZE 500 mg/kg significantly prevented the escalation of AI compared to DC and both the doses of ROSI treatment (p<0.05). Both 8 mg/kg and 16 mg/kg ROSI significantly prevented the rise in AI compared to DC alone. However, there was no significant difference between these doses (p>0.05) [Table/Fig-4].
Atherogenic index (AGI) in plain control and treatment groups.
Percentage Difference in the Thickness of Aorta among Study Groups from PC
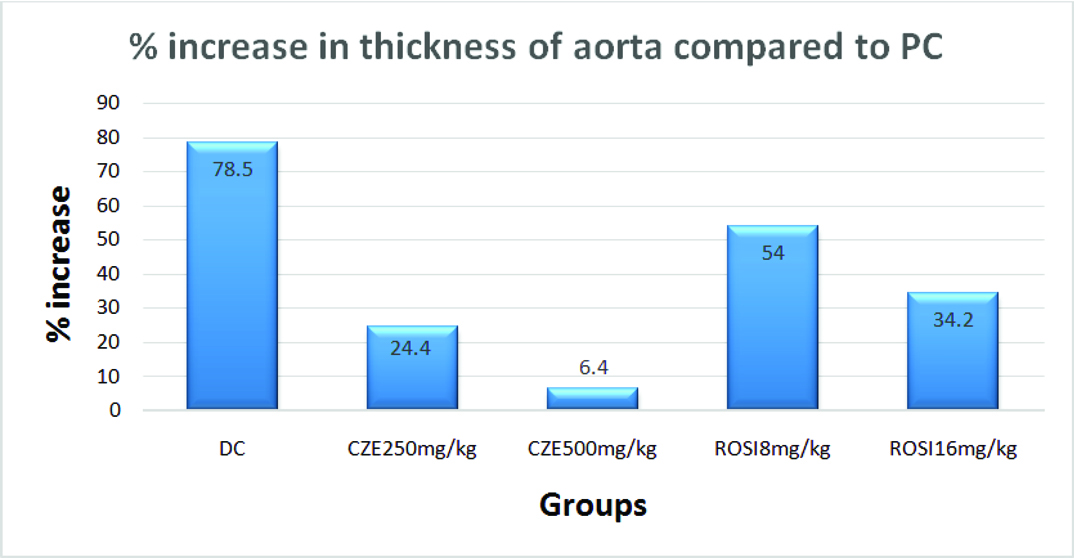
The aorta in dexamethasone treated group thickened by 78.5% whereas, ROSI 8 mg/kg and 16 mg/kg groups showed 34.2% and 54% thickness respectively. CZE 250 mg/kg group exhibited 24.4% increase in thickness of aorta and least percent increase in thickness observed in CZE 500 mg/kg with 6.4% compared to PC group [Table/Fig-5].
Percentage increase in thickness of aorta in study groups compared to Plain Control (PC).
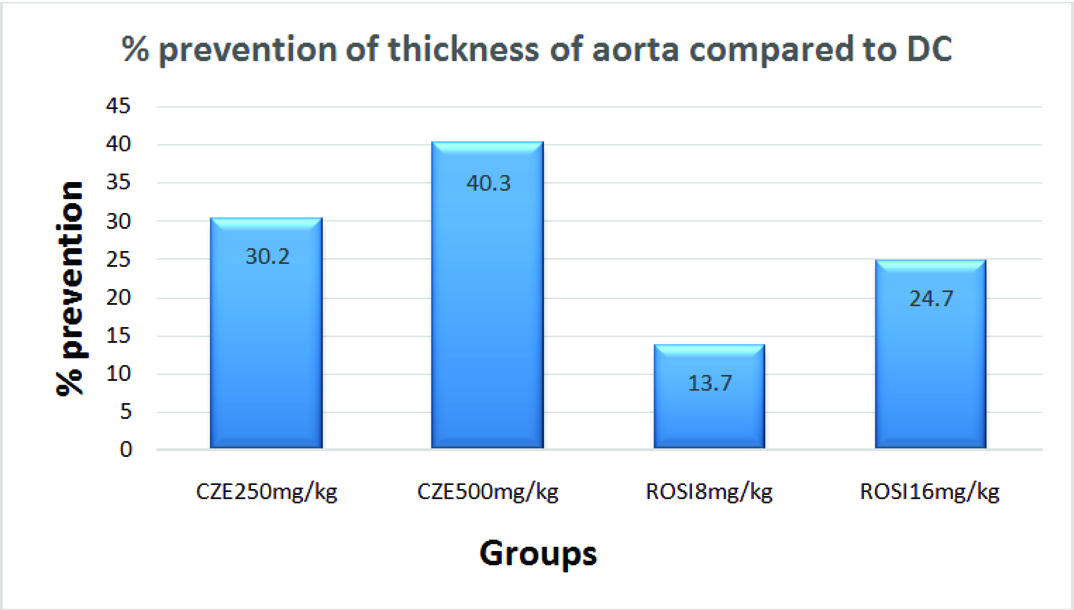
Percentage Difference in the Thickness of Aorta among CZE and ROSI Treatment Groups from DC
CZE 500 mg/kg effectively prevented the development of thickness of the aorta at maximum of 40.3% followed by CZE 250 mg/kg (30.2%) from DC group. ROSI 8mg/kg and 16mg/kg prevented the increase in thickness of aorta by 13.7% and 24.7% respectively from DC [Table/Fig-6].
Percentage difference in thickness of the aorta in study groups compared to Dexa Control (DC).
Histopathological Findings
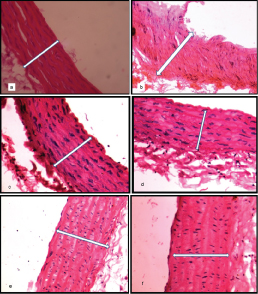
All sections of aorta of all groups were observed for the atherosclerotic changes and they were graded as mild, moderate and severe [Table/Fig-7]. The sections were observed under microscope with 400X magnification. Morphologically large sized artery consisting of inner tunica intima, middle tunica media and outer tunica adventitia. The tunica intima is lined by endothelial cells. The tunica media consisting of smooth muscle cells with elastic fibres with moderate thickening. The tunica adventitia is composed of loose connective tissue [Table/Fig-8a-e].
Interpretation of histopathological changes in the aorta on day 12 in study groups.
| Group n=6 | Atherosclerotic changes |
|---|
| PC | - |
| DC | +++ |
| ROSI 8 mg/kg | ++ |
| ROSI 16 mg/kg | + |
| CZE 250 mg/kg | - |
| CZE 500 mg/kg | - |
Note: (’-’= Absent, ’+’= Mild. ’++’= Moderate, ’+++’= Severe)
Photomicrographs of rats aorta captured under 400X magnification, Scale bar: 20 μm, H&E stain; a) Plain control (PC), normal aorta; b) Dexa control (dexamethasone 8 mg/kg) (DC) showing severe thickening of wall of aorta; c) CZE 250 mg/kg+ Dexamethasone 8 mg/kg showing normal appearance of wall of aorta; d) CZE 500 mg/kg+ Dexamethasone 8 mg/kg showing normal appearance of wall of aorta; e) ROSI 8 mg/kg+ Dexamethasone 8 mg/kg showing moderate thickening of wall of aorta; f) ROSI 16 mg/kg+ Dexamethasone 8 mg/kg showing mild thickening of wall of aorta.
Discussion
Several studies on dexamethasone induced insulin resistance model have also suggested the possible role of it in inducing dyslipidemia [19]. The current study is also in accordance with above mentioned statement as the rats developed marked dyslipidemia with 8 mg/kg of dexamethasone dosing for six days. In addition, this study focused on effects of steroid induced dyslipidemia on AI, an index to assess risk of atherogenicity, Cardiac Risk Ratio (CRR) and atherosclerotic changes in the vasculature of the aorta.
The data in this study revealed that the high dose of dexamethasone (8 mg/kg) significantly raised the AI as compared to control that may increase the risk of atherogenicity. CZE treatment proven its potential in preventing rise in AI compared to ROSI pre-treatment. It also prevented dyslipidemia evidenced by increased HDL and dampened TG, CH and LDL levels markedly against dexamethasone treatment. Microphotographs studied in CZE groups showed normal pattern of morphological architecture with no atherosclerotic changes. In CZE 500 mg/kg, the thickness of the aorta in the images [Table/Fig-8d] showed minimum of 6.4% increase compared to PC and prevented thickening of aorta maximum of 40.3% compared to DC. In this study, CZE treatments has proven its efficacy in preventing dyslipidemia, rise of AI and atherosclerotic change of aorta over both doses of ROSI treatment.
The lipid lowering effects of CZE in this study are in accordance with the review by Bandara T et al., as low lipid levels are associated with increased adiponectin levels [20]. A study by Wei Z et al., postulated that, treatment for four weeks with cinnmaldehyde, a major constituent form the C. zeylanicum has a role in markedly reducing blood levels of TG and LDL, and enhanced HDL and the LDL levels are much lower than MET treatment [21]. Besides, a study by Mahmood S et al., supports the above finding by stating that, administration of 20 mg/kg cinnamaldehyde reduces HbA1C, serum CH and TG levels markedly [22]. In present study, the findings are corresponding to the above mentioned. In support, a clinical trial by Khan A et al., found the improved lipoproteins in people with Type II DM [23]. In addition, 200 mg/kg of Cinnamon markedly reduced the levels of CH, TG and increased HDL-C compared to active control groups [24]. Ciftci M et al., put forward that administration of various concentrations of cinnamon oil reduced CH in chickens due to its hypolipidemic and anti-oxidative properties, and also polyunsaturated fatty acid ratio increased [25,26]. Interestingly, CZE treatment did not show marked effect in reducing VLDL compared to ROSI suggesting its minimal role in maintaining VLDL. This finding is supported by the work done by Sharafeldin K et al., [27]. With all above, it is coherent that the cinnamaldehyde, the main constituent might be responsible for hypolipidemic effects of CZE. Whereas, the beneficial effects of CZE in reducing risk of atherogenesis can be attributed to its positive effect on HDL cholesterol against dexamethasone treatment.
Dramatically, no atherosclerosis of aorta was noted with CZE treatment, possibly due to presence of abundant HDL and compensatory reduction in LDL levels and their peroxidation. It also has the action of inhibiting copper mediated LDL oxidation and Cholesteryl Ester Transfer Protein (CETP). As postulated by Jarvill-Taylor KJ et al., It is believed that the MHCP which claims its insulin mimetic property also may play crucial role in preventing atherosclerosis probably by reducing glucose uptake and increasing peripheral glucose uptake into cells. It also increases the glycogen synthesis primarily in the liver and muscle tissue [12]. These insulin sensitizing actions of MHCP could also be helpful in preventing the elevation of serum lipids and atherosclerosis. The favourable effects of CZE against steroid induced atherosclerosis could also be attributed to its strongest action against advanced glycation end products and anti-oxidant activity [28]. The elasticity and relaxation of arterial smooth muscle were well maintained with CZE treatment probably by release of Endothelium-Derived Relaxing Factor (EDRF) upon stimulation by Ach from tunica intima [29]. Further, CZE treatment markedly elevated HDL-C in rats, and might have prevented the steroid induced atherosclerosis [30]. Additionally, CZE treatment could have been supported in the maintenance of integrity of the vessel wall by inhibiting endothelin-1 which plays a key role in thickening of the vessel wall in insulin resistance like condition [31]. The CZE was employed and compared for its preventive effects with an insulin sensitizer like rosiglitazone in order to understand its role in steroid diabetes associated complications like dyslipidemia and atherosclerosis.
Limitation
The lipid lowering effect and anti-atherogenesis property was not compared with any other hypolipidemic agents.
Conclusion
To conclude, high dose of dexamethasone increases the risk of atherogenicity in blood vessels which can precede by their sclerosis evidenced by increased thickness of the aorta. However, the aqueous extract of C.zeylanicum bark extract has the potential to prevent steroid induced rise in atherogenic index and sclerosis of vessels in Wistar rats. Further, the effects of the active constituents’ cinnamaldehyde and MHCP have to be evaluated further for their efficacy and safety to develop them as a future agents, that are helpful in diabetes associated dislipidemia and also to prevent the sclerosis of blood vessels.
Abbreviations
TG- Triglycerides
HDL- High Density Lipoprotein
LDL- Low Density Lipoprotein
VLDL- Very Low Density Lipoprotein
ER- Endoplasmic Reticulam
GC- Glucocorticoid
Ach- Acetylcholine
CH- Cholesterol