Unusually Detected Anti-M Antibody Presenting as Cross Match Incompatibility in a Female Child Diagnosed with Small Round Cell Tumour
Sheetal Anand Chandak1, Aruna Vishwanath Vanikar2
1 Assistant Professor, Department of Immunohaematology and Transfusion Sciences, Institute of Kidney Diseases and Transplantation Sciences, Ahmedabad, Gujarat, India.
2 Professor and Head, Department of Cell Therapy and Regenerative Medicine, G.R. Doshi and K.M. Mehta Institute of Kidney Diseases and Research Centre and Dr. H.L. Trivedi Institute of Transplantation Sciences (IKDRC-ITS), Ahmedabad, Gujarat, India.
NAME, ADDRESS, E-MAIL ID OF THE CORRESPONDING AUTHOR: Dr. Sheetal Anand Chandak, A 401, Sankalp Sandipani Opp Gandhi Ashram Near Chandan Khadi Bhandar Subhash Bridge, Ahmedabad-300026, Gujarat, India.
E-mail: sheetalchandak29@gmail.com
MNS antigen system is one of the human blood group systems. Anti-M antibody is a relatively common, naturally occurring antibody of IgM variety. Clinically significant anti-M antibody is reactive at 37°C in the anti-human globulin phase due to high thermal amplitude of IgM component or presence of IgG component. If anti-M antibody is activated at 37°C or in the anti-human globulin phase, it may cause delayed haemolytic transfusion reactions or haemolytic disease of newborn, which suggest variable clinical significance. We report a case of an unusually detected anti-M antibody presenting as cross match incompatibility in a one-year-old female child with a lump in the right lumbar region, which was later diagnosed as small round cell tumour in the right kidney.
Agglutination, Blood group, Thermal amplitude
Case Report
A one-year-old female child of 12.4 kg body weight presented with complaints of abdominal pain since three months followed by fever without chills and rigors, on and off since seven days. She had no complains of cough, cold, diarrhea, vomiting etc. She was delivered full-term via vaginal delivery. She had no siblings, was immunized according to her age and had normal development. No other significant history was given by parents. On examination, her body temperature was normal, with good volume regular pulse (84/minute), normal blood pressure and capillary refilling time. Her respiratory, cardiovascular and central nervous system examination was unremarkable. Abdominal examination revealed 6x6 cm lump palpable in right lumbar region. It was firm and free from the overlying skin.
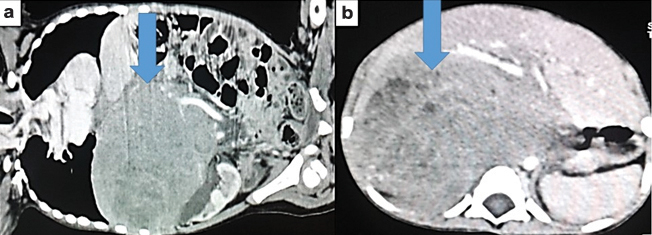
Laboratory investigations revealed haemoglobin, 8.3 gm/dl, total white blood cell count, 9x103/μl, platelet count, 3x105/μl, unremarkable coagulation profile, liver and renal function tests, serum electrolytes and blood sugar. Ultrasonography revealed a 7x7 cm mass involving upper and mid pole of right kidney, confirmed by computerized tomography scan. It revealed a right renal tumour in the upper pole measuring 0.9x9.3x7.9 cm with renal vein thrombosis and causing displacement of aorta, favouring malignant right renal mass [Table/Fig-1a,b].
(a and b): Right renal mass confirmed with computed tomography scan. Arrow denotes mass in the right kidney.
For diagnosis of tumour pathology, renal biopsy was planned under general anaesthesia. Requisition was sent to the blood bank for 15 ml/kg of Red Cells Concentrate (RCC). Routine Blood grouping was performed by solid phase technology (Immucor Galileo Assay). On complete forward and reverse grouping she was found to have blood group “B” ‘Rh-positive. No discrepancy in the grouping was observed. No agglutination was seen in “O” cells. However, cross-match by indirect Coomb’s test (Matrix Gel system AHG Coomb’s card) was incompatible with grade 4+ on testing with three different “B” positive RCC. Direct Coomb’s test using poly specific Anti-Human Globulin (AHG) reagents (anti-IgG and C3d) was negative and indirect Coomb’s test with pooled “O”cells was positive. Auto control was negative. Hence, she was subjected to screening for antibodies.
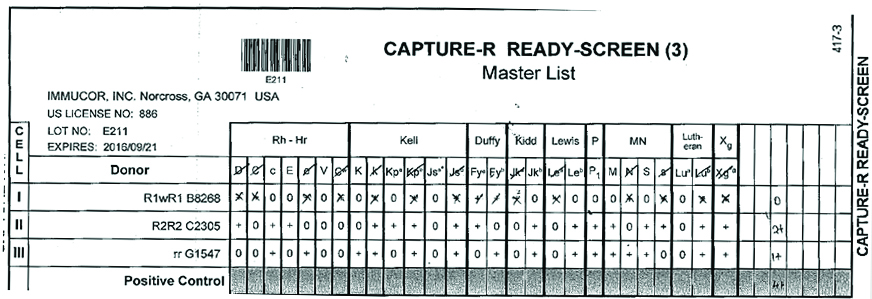
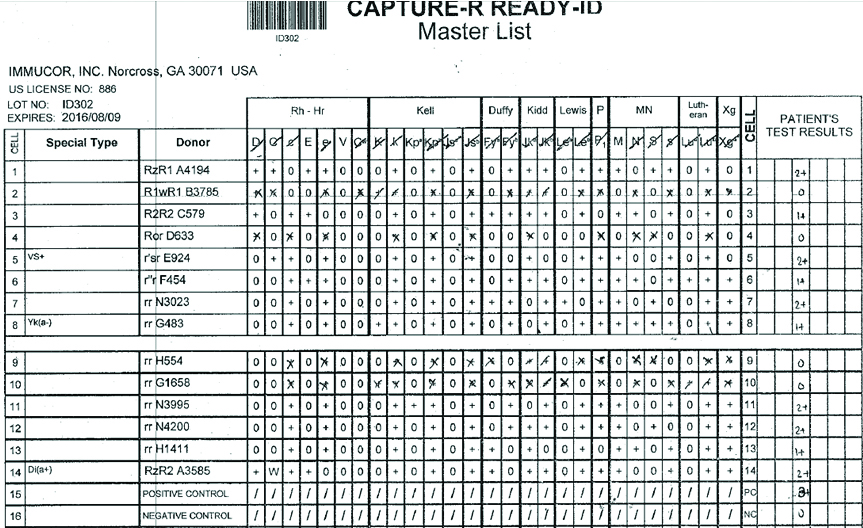
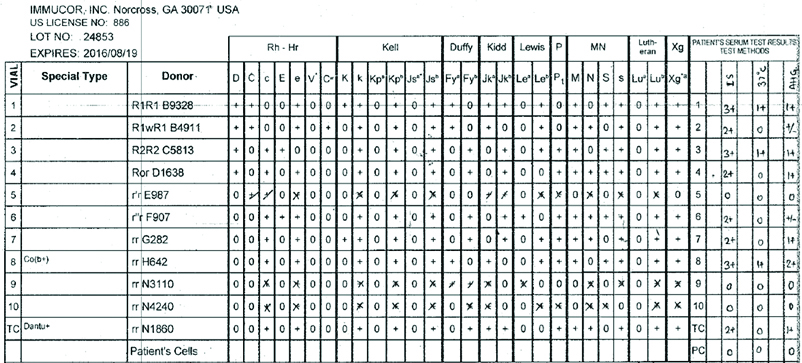
Three-cell panel antibody screening was performed on fully automated immunohaematology analyser (GALILEO Immucor, USA) using Capture R ready screen cells on Solid Phase Red Cell Adherence (SPRCA) technology [Table/Fig-2]. In three cell panel strength of the reaction is 2+ with homozygous (M+N-) cells and 1+ heterozygous (M+N+) cells. Fourteen cell panel (Immucor, GA, USA) screening was performed to exclude anti-c, anti-E, anti-K, anti-Kpa, anti-Jkb, anti-Leb, anti-M, anti-P1 and anti-S antibodies [Table/Fig-3]. Anti-E and anti-M antibodies were identified. The sample was then subjected to 11 cell panel for further confirmation by immediate spin, at 370C temperature, and with AHG [Table/Fig-4]. Anti-M antibody was identified on immediate spin at room temperature, suggestive of IgM class of antibodies. The test was also positive after incubation at 370C for 45 minutes by tube method suggestive of high thermal amplitude of IgM. The test was weakly positive with AHG, suggestive of some component of IgG class antibody also. Thus, the patient had anti-M allo-antibodies confirmed by negative report for minor antigen typing for M antigen. Thus, this reaction pattern of antibody screening and identification panel suggested presence of anti-M allo-antibody of IgM class reacting at higher thermal amplitude. Patient’s serum was cross matched with multiple units of “B” positive RCC and was eventually found to be compatible with M antigen negative unit.
Results of three- cell panel antibody screening for detection of antibodies (+) Positive (-) Negative result for the corresponding antigen.
Antibody identification by 14 cell panel.
11 cell panel after (1) immediate spin, (2) at 370 C temperature, and (3) with anti human globulin.
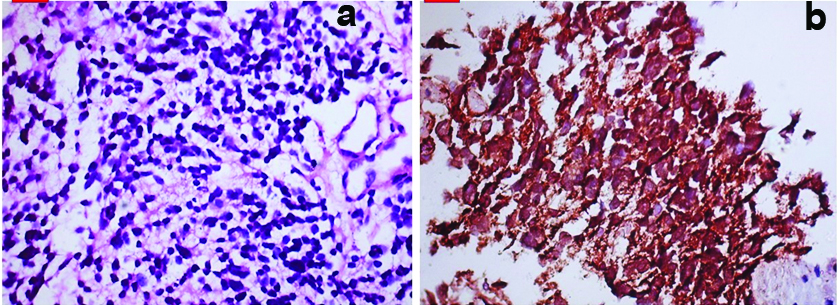
The renal biopsy of patient was performed under general anaesthesia from right kidney under aseptic precautions. Post renal biopsy course was uneventful. She did not require any blood transfusion. The histopathology of right renal mass showed small round cells having inconspicuous cytoplasm and round nuclei, suggestive of small round cell tumour [Table/Fig-5a,b]. On immunohistochemistry tumour cells were positive for CD99, Vimentin, Non-specific esterase and negative for CD20, S100, CD3, WT1, suggestive of Primitive neuroectodermal tumour. She was referred to Oncology services at different hospital for further management.
a) Histopathology revealed tumour cells having inconspicuous cytoplasm and round darkly stained nuclei in image (H&E, 40X); b) Tumour cells were positive for NSE (IHC, 40X).
Discussion
Anti-M antibodies naturally occurring cold reactive and are usually clinically insignificant [1]. Majority of these antibodies are of IgM class. They are inactive at 37°C and discrepancy encountered can be resolved by performing the tests at warm temperatures [2-4]. Rarely, Anti-M antibody can be reactive at 37°C and in the antihuman globulin phase. In such unusual cases, the interpretation must be made cautiously. These antibodies become significant clinically and can cause haemolytic transfusion reactions and Haemolytic disease of newborn. Tandon R et al., reported two cases of clinically significant anti-M antibodies of which, one was immunizing type reactive at 37°C as well as AHG phase with IgG component; and the other was naturally occurring antibody reactive below 37°C. The incidence of anti-M antibodies in the donor cells was 1 in 2500 in homozygous (M+N-) cells and 1 in 5000 with heterozygous (M+N+) cells [5]. Since our patient did not have any past history of transfusion the antibody was naturally occurring antibody in this case and not immunizing type of antibody. Shah OP et al., reported frequency of anti-M antibodies in their study population to be 13.98% [6]. In their study, all cases of anti-M antibody were biphasic in nature. They were reactive at 37°C and were clinically significant as they had the potential to cause HDN [6-9].
Our patient had anti-M antibody of IgM class with clinical significance reacting at high thermal amplitude and also with some component of IgG. False positive reactions can be ruled out by strict warm testing conditions. According to our knowledge this is the first report of presence of anti-M antibody with small round cell tumour of right kidney. This case highlights the significance of performing multi-panel antibody testing to detect uncommon antibodies such as anti-M antibody, and prevent adverse transfusion reactions.
Conclusion
Anti-M antibody cannot be ignored especially when it is reactive at 37°C. Patient should be transfused with M negative packed cells in the presence of such antibodies whenever blood transfusion will be required.
[1]. Reid ME, Westhoff CM, Other blood group antigens and antibodies. In: Hillyer C, Anderson K, Silberstien L, Ness P, Roback JD, editorsBlood Bank. Transfus. Med 2009 2nd edNew DelhiChurchill Livingstone:96-97. [Google Scholar]
[2]. Khalid S, Dantes R, Varghese S, Al Hakawati I, Naturally occurring anti M complicating ABO groupingIndian J Pathol Microbiol 2011 54:170-72. [Google Scholar]
[3]. Brecher ME, AABB Technical Manual 2005 14th edBethesdaAmerican Association of Blood Banks [Google Scholar]
[4]. Steinert JN, Kirkegaard J, Patel Jigar S, Unusual antibody reactions in a 55-year-old manLab Med 2006 37:671-74. [Google Scholar]
[5]. Tandon R, Kataria R, Chaudhry R, Anti M: Report of two cases and review of literatureAsian J Transfus Sci 2008 2:81-83. [Google Scholar]
[6]. Shah SP, Kalgutkar SM, Sawant RB, Deshpande AS, Anti M antibodies: Biphasic (reactive at room temperature and at 37°C) A case seriesAsian J Transfus Sci 2016 10:159-60. [Google Scholar]
[7]. Alperin JB, Riglin H, Branch DR, Gallagher MT, Petz LD, Anti-M causing delayed haemolytic transfusion reactionTransfusion 1983 23:322-24. [Google Scholar]
[8]. Sancho JM, Pujol M, Fernandez F, Soler FM, Manzano P, Felio E, Delayed haemolytic transfusion reaction due to anti M antibodyBr J Haematol 1998 103:268-69. [Google Scholar]
[9]. Duguid JK, Bromilow IM, Entwistle GD, Wilkinson R, Haemolytic disease of the newborn due to anti MVox Sanguinis 1995 68:195-96. [Google Scholar]