Neurocysticercosis (NCC), a neglected tropical disease is one of the leading aetiologies of epilepsy and neurological morbidity worldwide [1,2]. The disease is considered to be endemic in developing countries such as Latin America, China, South-East Asia, Indonesia, Haiti and Sub-Saharan Africa [3-5]. With increased trends in Globalisation, the disease is being spread worldwide among developed countries and deaths have also been reported from countries like United States [6,7]. Though the global frequency of NCC is currently not well explored, a study suggests that 1.7–3.0 million people have been estimated to have accquired epilepsy attributable to NCC which contributes to significant morbidity [8]. The Indian scenario shows that disease is spread out all throughout the country with the exception of Kashmir and Kerala [9,10].
NCC is a severe manifestation of helminthic disease in humans resulting from ingestion of Cysticercus cellulosae the larval form of Taenia solium by inadvertent faeco-oral contamination [2]. These larval oncospheres have predilection to different sites like brain, eyes, heart, skeletal muscles and cutaneous regions among which, the most predominant site is the brain and leads to secondary manifestations in humans [11]. NCC is documented in both adults and children presenting with cysts varying from single to multiple, either parenchymal or racemose (subarachnoid) or intraventricular cysts or chronic cysticercal meningitis with basal exudates, or hydrocephalus with parenchymal cysts frequently leading to calcifications [8]. NCC is not a fatal by itself except when it presents rarely with indolent subacute or chronic meningitis, but the morbidity associated with it is high and affects the quality of living. Epidemiology data regarding the prevalence of NCC and its pathogenesis profile has been found helpful by the clinicians in assisting the diagnosis and management of the infected individuals [12]. Only a few reports are available on the recent prevalence of NCC recently in India, especially in the Southern regions and specifically among Tamil population [9,10,13-16].
The conventional diagnostic method of NCC is a knotty process based on neuroimaging and serological methods but at times highly invasive histopathological demonstration of the cyst in the biopsied tissue is necessitated. The major drawback for assessing the disease burden of NCC is the lack of proper ‘gold standard’ tests for screening as well as detecting carriers and asymptomatic individuals. Despite which, with different study settings refers either visualization of scolex in ring lesions by Magnetic Resonance Imaging (MRI) or Computed Tomography (CT), anatomopathologic study i.e., histologic demonstration of parasites in biopsy are considered standard [17-19]. But, being a highly invasive procedure histologic demonstration is not feasible also imaging techniques are not only unavailable in all resource limited settings but also diagnosis solely based on it are not NCC as they may be any other cystic lesions or tuberculoma [19]. Sero-diagnostic techniques like Enzyme-linked Immune Electro Transfer Blot (EITB), Enzyme-Linked Immune Sorbent Assay (ELISA) have evoked a considerable interest in the diagnosis of NCC. But, EITB is considered to have the higher specificity and sensitivity, Hence, it is recommended by Centers for Disease Control and Prevention (CDC) for NCC diagnosis [20]. EITB has been reported to offer a better range of both specificity and sensitivity varying approximately from 70% to 90% [21]. Diagnosis made by serological methods as a stand – alone test has limited value; so it has to be compared along with imaging data and patient history. The standard classification of diagnostic certainity given by OH Del Brutto aids in this process [22]. Our study was carried out to assess the clinical parameters of NCC suspected individuals along with imaging methods and Immunological test for diagnosis of NCC. This study is first of its kind carried out in this study setting in associating the varied clinical manifestations of NCC with serological and imaging findings.
Materials and Methods
Ethical approval obtained from the Institute Ethical Committee of JIPMER, Puducherry, India, to conduct the study (IEC/SC/2012/4/142). Individuals who participated in the study were well informed about the purpose of the study and written informed consent obtained in their regional language. In the case of minors, parent’s/guardian’s provided informed consent on their behalf.
The study includes patients suggestive for NCC based on clinical manifestations or radiological manifestations like cystic lesions or granuloma majorly from neurology, radiology and medicine departments including age groups of five years and above and both genders with an exception of pregnant women to avoid additional trauma to them. Selection of patients was carried out based on the presence of seizures and any cystic lesions or granuloma in CT/MRI scan. A detailed history was documented for the cases during August 2012 to December 2015 (n=119 cases) [Table/Fig-1] and the different aetiological factors and symptoms were analysed. This includes the medical history, food habits, meat consumption, history of passing worms, seizure semiology and frequency seizures, family history of seizures, results of neuro-imaging studies and treatment underwent.
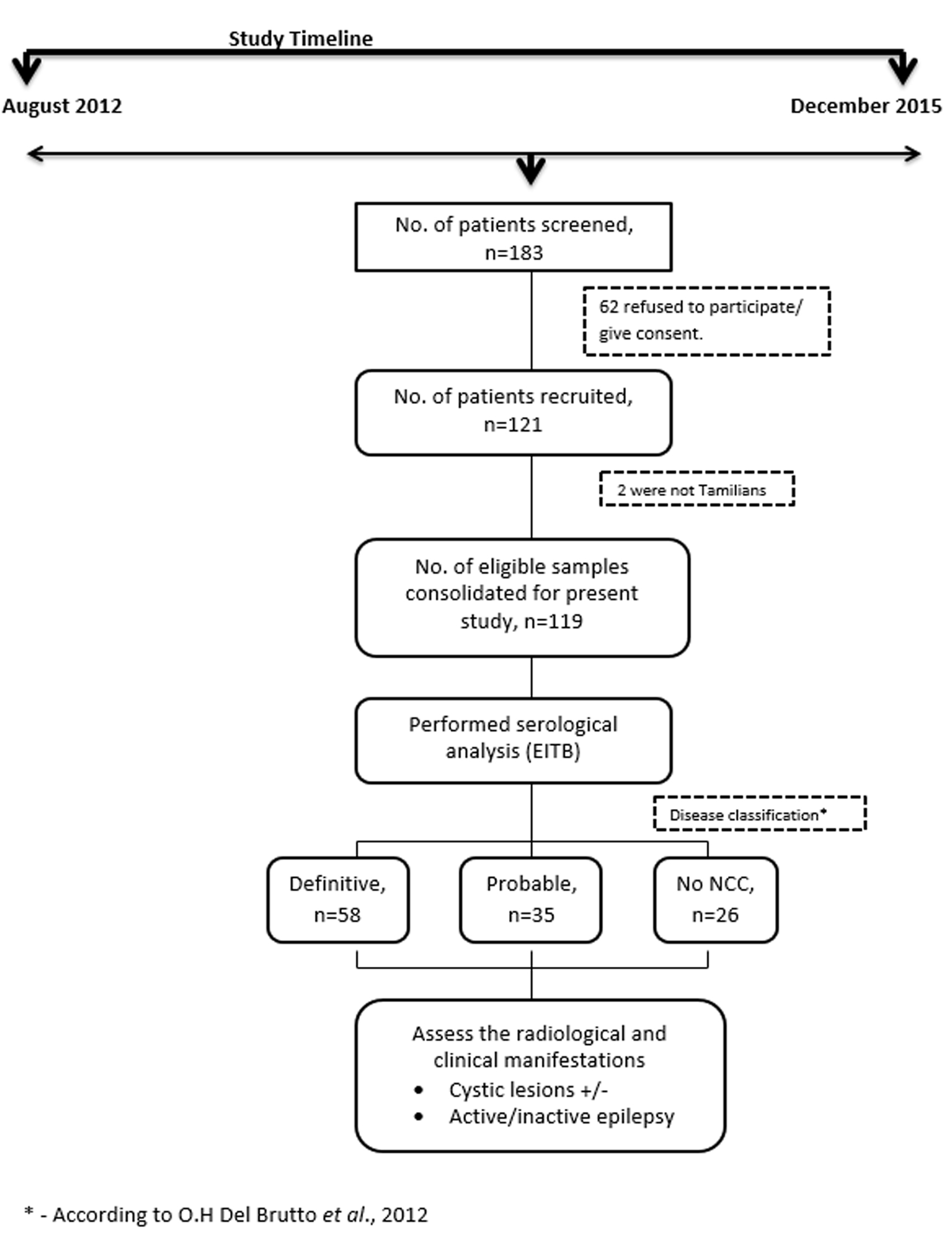
The clinical symptoms and presentations were assessed on the detailed proforma from clinically and radiologically suspected cases of NCC, with informed consent. Epileptic patients were classified based on the duration of seizures, where Patients with Active Epilepsy (PWAE) were those having seizures within past two years or undergoing anti-epileptic medication and cases unfulfilling the above criteria were Patient with Inactive Epilepsy (PWIE) as described elsewhere [23]. Based on the origin of seizures and as per epilepsy classification by International League Against Epilepsy (ILAE), they are classified as generalized and focal [24].
Radiological Screening
The neuro-imaging findings of CT/MRI scan reports of the study subjects were documented. Based on which the classification was carried out depending on the number of cysts found in imaging as either single or multiple lesions. Active (appearance of scolex of cyst in imaging as hypo/hyperdense or hypo/hyperintense lesion without contrast enhancement), transitional (presence of a rim or ring enhancing lesion) and inactive (calcified granuloma) are classified considering the viability of the parasite as proposed elsewhere [25].
Serological Screening
Venous blood samples (2 ml) were collected and processed by allowing it to stand for 15-30 minutes at room temperature followed by centrifugation at 1520 X g for 10 minutes and stored the serum (supernatant) at -20ºC until further use. All the samples of the suspected cases were subjected to the serological test for NCC. The results were documented, and the seropositivity rate was calculated among the patients.
EITB was carried out with slight modification from an earlier study [26]. In brief, cyst metacestode antigen was resolved in 10% Sodium Dodecyl Sulphate-Polyacrylamide Gel Electrophoresis (SDS-PAGE) along with the protein markers of standard molecular weight (Thermo Scientific, India) [27]. The gel was electro-blotted by the semi-dry method to nitro-cellulose membrane followed by reversible staining with Ponceau S to cut strips and blocked with Bovine Serum Albumin (BSA). The patient sample (primary) and rabbit anti-human IgG secondary antibody conjugated with horseradish peroxidase was supplemented to the membrane and the reactivity of the antibody was observed after the addition of diaminobenzidine (DAB) substrate solution (Sigma-Aldrich, USA). The immunoreactive glycoprotein bands observed predominantly around 20-25 kDa, 39-42 kDa, 50 kDa were considered as positive serology.
Diagnostic Certainty of NCC
Classification of the study population based on the revised degree of certainty [22], diagnosis of NCC, resulted in four different criteria: i) Absolute; ii) Major; iii) Minor; and iv) Epidemiological. In the study, we chose the absolute criteria based on, the existence of cystic lesions revealing the scolex on neuroimaging studies. The major criteria encompassed lesions indicative of neuro-cysticercosis or serology positive by serum immunoblotting for cysticercus antibodies or resolution of the cystic lesions with anti-parasitic treatment or spontaneous resolution of small single enhancing lesions [22]. The minor criteria included strong clinical manifestation suggesting NCC or lesions compatible with cysticercosis or positive serology by ELISA. Since, India is considered to be endemic to NCC, our study participants fulfilled the criteria [2]. Then the combination of these criteria suggested diagnosis as definitive (presence of one absolute criterion or with two major criteria plus one minor and one epidemiologic criterion) and probable (presence of one major plus two minor criteria or else one major plus one minor and one epidemiologic criterion or with three minor plus one epidemiologic criterion) [22]. The cases that do not fall in the above criteria were classified as no NCC.
Statistical Analysis
All data were documented and processed in Microsoft Excel. Pivot tables were generated, and the data were obtained as percentages.
Results
Demographic Data
A detailed proforma was collected from 119 (51 female and 68 male) clinically suspected cases [Table/Fig-2]. The study population includes different age groups with an average paediatrics age of 10.4 years and adults with 36.8 years age range. The majority of the study population was from Villupuram district (36.1%), followed by Tiruvannamalai (20.2%), Cuddalore (18.5%) and Puducherry (10.9%). A few of them were from other districts like Thanjavur, Ariyalur, Salem, Nagapattinam, Perambalur, Nammakal, Trichy, Krishnagiri, Kalakurichi and Kanchipuram.
Patient demographic details.
| Parameters* | Total n(%) | Serology Positive n(%) | Multiple lesions Positive n(%) | Single lesions Positive n(%) |
|---|
| Gender: |
| Male | 68 (57.1) | 45 (66.2) | 25 (58.1) | 18 (41.9) |
| Female | 51 (42.9) | 32 (62.7) | 15 (42.9) | 20 (57.1) |
| Age: |
| Adult | 101 (84.9) | 65 (64.4) | 35 (56.5) | 27 (43.5) |
| Paediatric | 18 (15.1) | 12 (66.7) | 5 (31.2) | 11 (68.8) |
| Dietary habits: |
| Vegetarian diet | 13 (10.9) | 5 (38.5) | 2 (33.3) | 4 (66.7) |
| Non-vegetarian diet | 106 (89) | 72 (67.9) | 38 (52.8) | 34 (47.2) |
| Raw vegetable consumption | 70 (58.8) | 43 (61.4) | 21 (45.7) | 25 (54.3) |
| Pork consumption | 69 (58.0) | 36 (52.2) | 19 (51.4) | 18 (48.6) |
| Influential environmental factors: |
| Bowel Movements (O) | 84 (70.5) | 55 (65.5) | 33 (56.9) | 25 (43.1) |
| Bowel Movements (T) | 35 (29.4) | 22 (62.9) | 7 (43.7) | 9 (56.3) |
| Pig Rearing | 29 (24.3) | 18 (62.1) | 10 (58.8) | 7 (41.2) |
| Patient History: |
| H/o Taeniasis or passing worms | 36 (30.3) | 23 (63.9) | 8 (32) | 17 (68) |
| H/o cysticercosis | 7 (5.9) | 5 (71.4) | 4 (57.1) | 3 (42.9) |
| clinical Parameter: |
| PWAE | 82 (68.9) | 52 (63.4) | 29 (51.8) | 27 (48.2) |
| PWIE | 6 (5.0) | 3 (50) | 2 (33.3) | 4 (66.7) |
Data expressed as n (%); (O) – Open field; (T) – Toilet
PWAE-patients with active epilepsy
PWIE-patients with inactive epilepsy
Clinical Manifestations
Out of 119 cases 73.9% (n=88) presented with epilepsy, among them 93.2% (n=82) had active epilepsy (PWAE) and 6.8% (n=6) had inactive epilepsy (PWIE). And among the patients with a history of seizures, 52% of cases had multiple episodes of seizures, and 48% had only single episode. In addition to this other co-morbid conditions documented were stratified based on serological and imaging findings [Table/Fig-3]. Among the study group, 48.7% (n=58) were with definitive NCC, 29.4% (n=35) were probable NCC, and 21.9% (n=26) were having no NCC based on Del Brutto’s diagnostic criteria.
Clinical manifestations based on Del Brutto classification.
| Parameters | definitive Nccn (%) | Probable NCC n (%) | No NCC n (%) |
|---|
| Serology |
| Positive | 54 (45.4) | 19 (16.0) | 4 (3.4) |
| Negative | 4 (3.4) | 16 (13.4) | 22 (18.5) |
| Seizures |
| Focal | 25 (21) | 7 (5.9) | 3 (2.5) |
| Generalized | 19 (16) | 21 (17.6) | 13 (10.9) |
| No | 14 (11.8) | 7 (5.9) | 10 (8.4) |
| Chronic headache |
| Headache | 22 (18.5) | 12 (10.1) | 8 (6.7) |
| Headache and Vomiting | 23 (19.3) | 14 (11.8) | 9 (7.6) |
| No | 13 (10.9) | 9 (7.6) | 9 (7.6) |
| Lesions |
| No | 2 (1.7) | 20 (16.8) | 23 (19.3) |
| Yes | 56 (47.1) | 15 (12.6) | 3 (2.5) |
| Type of epilepsy |
| Active | 41 (34.5) | 25 (21.0) | 16 (13.4) |
| Inactive | 3 (2.5) | 3 (2.5) | 0 |
| No | 14 (11.8) | 7 (5.9) | 10 (8.4) |
| No. of Lesions |
| Multiple | 31 26.1) | 8 (6.7) | 0 |
| Single | 27 (22.7) | 9 (7.6) | 6 (5.0) |
| No | 0 | 18 (15.1) | 20 (16.8) |
Serological Findings
Serological tests results revealed 64.7% of the cases (66.2% of male and 62.7% were female) positive of NCC serology and 35.3% showed negative serology in antibody detection by EITB. The seropositivity rate among adult and paediatric patients were found to be 64.4% and 66.7% respectively.
The most predominant clinical manifestations based on seropositivity are seizures (46.2%), chronic episodic headaches (22.7%) and headache with vomiting (26.0%). The other manifestations like muscle weakness (37.8%), neck pain (31.1%), blurred vision (29.4%), altered sensorium/abnormal behaviour (26.9%), hemiparesis/hemisensory loss (22.7%), cognitive deficits (21.9%), movement disorder (21.0%) and aphasia (21.0%) were found with higher seropositive correspondingly. And minor clinical manifestations based on seropositivity were found for pallor (14.3%), paraparesis (10.9%), meningeal irritation (8.4%), subcutaneous swelling (7.6%), cranial nerve palsy (6.7%) and pappiloedema (4.2%).
Neuroimaging Analysis
Among the study population of 119 cases, 80 cases had CT only 24 cases had MRI only and 15 cases with both CT as well as MRI imaging results. The presence of the cysts visualized by CT/MRI scans in 42% male and 38% females. Depending on the disease activity, 45.7% of the cases had calcified stages of cysts, 34% in a transitional stage and 20.2% in the active stage. While comparing the imaging and serological tests for diagnosis of NCC, it was observed that, around 46.2% of cases were positive by both serology and imaging whereas, 16% of cases positive by imaging were found to be seronegative. However, 18.5% of the cases negative for the presence of cysts by imaging techniques were seropositive while 19.3% of the cases were negative for both serology and radio imaging. The correlation of radio imaging results with presence and number of cysts along with serology results are shown in [Table/Fig-4]. Based on the location of the cysts it was found that most cases presented with cysts in the frontal lobes and parietal lobes, followed by other regions like occipital and temporal regions.
Stratification of clinical manifestations based on serology and imaging findings.
| Clinical Manifestations | Total | EITB | Imaging |
|---|
| Positive n (%) | Negative n (%) | Presence of Lesion | Absence of Lesion n (%) |
|---|
| total n (%) | Multiple n (%) | Single n (%) |
|---|
| Seizures | 88 | 55 (46.2) | 33 (27.7) | 62 (52.1) | 31 (50.0) | 31 (50.0) | 26 (29.5) |
| Generalized seizures | 53 | 30 (25.2) | 23 (19.3) | 31 (26.1) | 14 (45.2) | 17 (54.8) | 22 (41.5) |
| Focal seizures | 35 | 25 (21.0) | 10 (8.4) | 31 (26.1) | 17 (54.8) | 14 (45.2) | 4 (11.4) |
| No | 31 | 22 (18.5) | 9 (7.6) | 16 (13.5) | 9 (56.3) | 7 (43.8) | 15 (48.4) |
| Headache | 42 | 27 (22.7) | 15 (12.6) | 31 (26.1) | 16 (51.6) | 15 (48.4) | 11 (26.2) |
| Headache/Vomiting | 46 | 31 (26.0) | 15 (12.6) | 28 (23.5) | 17 (60.7) | 11 (39.3) | 18 (39.1) |
| No | 31 | 19 (16.0) | 12 (10.1) | 19 (16.0) | 7 (36.8) | 12 (63.2) | 12 (38.7) |
| Blurred vision | 56 | 35 (29.4) | 21 (17.7) | 35 (29.4) | 17 (48.6) | 18 (51.4) | 21 (37.5) |
| No | 63 | 42 (35.3) | 21 (17.7) | 43 (36.1) | 23 (53.5) | 20 (46.5) | 20 (31.7) |
| Subcutaneous swelling | 14 | 9 (7.6) | 5 (4.2) | 9 (7.6) | 4 (44.4) | 5 (55.6) | 5 (35.7) |
| No | 105 | 68 (57.1) | 37 (31.1) | 69 (58.0) | 36 (52.2) | 33 (47.8) | 36 (34.6) |
| Aphasia | 50 | 25 (21.0) | 25 (21.0) | 26 (21.9) | 14 (53.8) | 12 (46.2) | 24 (48.0) |
| No | 69 | 52 (43.7) | 17 (14.3) | 52 (43.7) | 26 (50.0) | 26 (50.0) | 17 (24.6) |
| Paraparesis | 20 | 13 (10.9) | 7 (5.9) | 9 (7.6) | 4 (44.4) | 5 (55.6) | 11 (55.0) |
| No | 99 | 64 (53.8) | 35 (29.4) | 69 (58.0) | 36 (52.2) | 33 (47.8) | 30 (30.3) |
| Neck pain | 59 | 37 (31.1) | 22(18.5) | 37 (31.1) | 20 (54.1) | 17 (45.9) | 22 (37.3) |
| No | 60 | 40 (33.6) | 20 (16.8) | 41 (34.5) | 20 (48.8) | 21 (51.2) | 19 (31.7) |
| Muscle weakness | 74 | 45 (37.8) | 29 (24.4) | 45 (37.8) | 21 (46.7) | 24 (53.3) | 29 (39.2) |
| No | 45 | 32 (26.9) | 13 (10.9) | 33 (27.7) | 19 (57.6) | 14 (42.4) | 12 (26.7) |
| Papilloedema | 7 | 5 (4.2) | 2 (1.7) | 3 (2.5) | 2 (66.7) | 1 (33.3) | 4 (57.1) |
| No | 112 | 72 (60.5) | 40 (33.6) | 75 (63.0) | 38 (50.7) | 37 (49.3) | 37 (33.0) |
| Meningeal irritation | 20 | 10 (8.4) | 10 (8.4) | 7 (5.9) | 4 (57.1) | 3 (42.9) | 13 (65.0) |
| No | 99 | 67 (56.3) | 32 (26.9) | 64 (53.8) | 36 (56.3) | 28 (43.8) | 35 (35.4) |
| Cognitive deficits | 46 | 26 (21.9) | 20 (16.8) | 21 (17.7) | 9 (42.9) | 12 (57.1) | 25 (54.3) |
| No | 73 | 51 (42.9) | 22 (18.5) | 57 (48.0) | 31 (54.4) | 26 (45.6) | 16 (21.9) |
| Cranial nerve palsy | 19 | 8 (6.7) | 11 (9.2) | 11 (9.2) | 4 (36.4) | 7 (63.6) | 8 (42.1) |
| No | 100 | 69 (58.0) | 31 (26.1) | 67 (56.3) | 36 (53.7) | 31 (46.3) | 33 (33.0) |
| Movement disorder | 41 | 25 (21.0) | 16 (13.5) | 22 (18.5) | 11 (50.0) | 11 (50.0) | 19 (46.3) |
| No | 78 | 52 (43.7) | 26 (21.9) | 56 (47.1) | 29 (51.8) | 27 (48.2) | 22 (28.2) |
| Pallor | 30 | 17 (14.3) | 13 (10.9) | 17 (14.3) | 5 (29.4) | 12 (70.6) | 13 (43.3) |
| No | 89 | 60 (50.4) | 29 (24.4) | 61 (51.3) | 35 (57.4) | 26 (42.6) | 28 (31.5) |
| Altered sensorium (AS) | 25 | 19 (16.0) | 6 (5.0) | 14 (11.8) | 8 (57.1) | 6 (42.9) | 11 (44.0) |
| Abnormal behavior (AB) | 6 | 4 (3.4) | 2 (1.7) | 2 (1.7) | 1 (50.0) | 1 (50.0) | 4 (66.7) |
| Both AS/AB | 20 | 9 (7.6) | 11 (9.2) | 12 (10.1) | 6 (50.0) | 6 (50.0) | 8 (40.0) |
| No | 68 | 45 (37.8) | 23 (19.3) | 50 (42.0) | 25 (50.0) | 25 (50.0) | 18 (26.5) |
| Hemiparesis (HP) | 3 | 3 (2.5) | 0 (0) | 3 (2.5) | 1 (33.3) | 2 (66.7) | 0 (0) |
| Hemisensory loss (HS) | 13 | 10 (8.4) | 3 (2.5) | 8 (6.7) | 3 (37.5) | 5 (62.5) | 5 (38.5) |
| Both HS/HP | 32 | 14 (11.8) | 18 (15.1) | 21 (17.7) | 10 (47.6) | 11 (52.4) | 11 (34.4) |
| No | 71 | 50 (42.0) | 21 (17.7) | 46 (38.7) | 26 (56.5) | 20 (43.5) | 25 (35.2) |
Discussion
NCC is one of the endemic parasitic diseases prevailing in various regions of India. As per WHO report, it is estimated to cause 29% of epilepsy cases in the endemic countries. Being a neglected tropical disease, it attracts less interest among the clinicians and researchers. Hence, only a few studies have been carried out from different provinces in India, but most of them clearly suggest the higher incidence of the disease spread [13-16,28,29]. The clinical manifestations of NCC are presented mainly by the parasite’s mass effect and the host-immune response built up against the parasite [6]. Our study population included Tamilians covering 13 districts of Tamil Nadu state and Puducherry in India. The study helps in exploring the myriad of clinical manifestations presented by suspected cases of NCC and involving the combinations and importance of two different diagnostic methodologies viz., serology and imaging techniques.
The overall gender wise seroprevalence of NCC showed a higher seroprevalence for males than that of females, which is in concordance with that of studies conducted in California [30]. Indeed, there are other studies which have demonstrated that young females are more affected [30]. Though gender may not play a role in the NCC disease causal, the reason of varying seroprevalence based on gender is ambiguous. As observed in our study, male patients who presented with the onset of the seizures or other symptoms of NCC attended our hospital at the early stages, whereas the female patients had already attended Primary Health Centre and had undergone anti-epileptic therapy/anti-parasitic therapy. They would report to tertiary care hospitals only when they encounter repeated episodes and/or a complicated course of epilepsy, which may be the possible reason for the varying levels of seropositivity based on gender distribution.
In our study, among the children studied seroprevalence of NCC was observed around 66.7% (12 positive out of 18 cases) whereas, it was 64.4% (65 out of 101 cases) in adults which is being in line with other similar studies on paediatric NCC cases from India and other regions [31-34]. The paediatric mean age group observed in our study is concordant to that reported from Eastern India, over a period of three years [35]. Children are likely to get more exposed to the contaminated environment or improper sanitary practices and have high chances of contracting any infestation of parasites in the gastrointestinal tract leading to cyst localization. But the total number of children included in the study is less than adults. This has to be confirmed further with a higher number of study individuals.
Among the various aetiological causes leading to NCC, environmental factors play a significant role in the distribution of the disease. In our study, as most of the people residing along the pig rearing communities under poor sanitation conditions were found positive for NCC and also most of the people who used the open field for bowel movements were seropositive. Hence, it is observed that open field defecation and poor sanitation of the surroundings would add to the source of infection and play a vital role in acquisition and spread of NCC along with other causative factors.
Based on the different diagnostic tools for NCC such as neuro-imaging and serological tests, revealed that most of the NCC patients were positive by both the methods. However, there were cases, where patients showed positive by imaging and negative by serology and also vice versa. Possible reason for this discrepancy can be due to the presence of very low levels of antibody/antigen titer detectable by serological tests or if the cyst is at the earlier stages of transition or has undergone calcification or cystic lesions due to other parasitic or microbial infections [28]. On the other hand, positivity by serology only could be encountered where cysts would resolve spontaneously or as the result of anti-parasitic therapy [36], which is being referred as “disappearing CT lesion”, or “vanishing lesion” as stated elsewhere [37]. In few cases, false positivity by serological tests may also be possible because of cross-reactivity. Irrespective of the location and number of the cysts present, the seropositivity levels remained unaltered. In our study, multiple lesions were higher than that of a single lesion, which is contradictory with many other single lesion study reports from India [37,38].
Around three-quarters (88 out of 119) of the study population was presented with seizures or epilepsy. This is being consistent with many other similar studies stating the frequency of epilepsy among symptomatic NCC cases ranging from 70%-90% [32]. When considering the serology and imaging positivity it accounts for 46% and 49% respectively. Based on the origin of seizures, though generalized pattern of seizure remains the most common type seen among the study subjects, more serology positive were seen in patients with focal seizures (partial seizures), which is in concordance with a study carried out in Odisha [31]. The second most common manifestation seen in our study was headaches with and without nausea which is again the more consistent manifestation and typical for NCC. There are only a few studies describing the psychiatric and altered sensorium features in NCC cases. This study helps in providing the measure of varied clinical manifestations presented with NCC patients in our study region. Also, the study sheds some light on the lifestyle parameters of the study group, and its association with serology and imaging findings. This would be indispensible for identifying various exposure risk factors leading to NCC. These factors are to be considered while carrying out surveillance/field studies in assessing the exposure status of taeniasis and or cysticercosis in India.
Limitation
Our study has its limitation like, being a single-centered hospital based study with limited sample size and has a bias towards the study population, so that it may not represent the overall community. The association of clinical impact on the disease condition could not be obtained due to a fewer subgroup population in the studied clinical parameters. Few parameters like intra-cranial pressure were not monitored in our study population. However, they play roles in the disease manifestation, which could be demonstrated with more stratified sample analysis with a large set of population.
Conclusion
The outcome of our study will assist in ascertaining the disease manifestation of NCC infection in the Tamilian population. This study helps in understanding the impact of varied range of neurological and neuro-psychiatric manifestations presented with NCC. Also, it is suggested that these manifestations are need to be born in mind when confronting with possible NCC cases especially from the endemic regions of the disease. It assists the clinicians in understanding the implications of diagnostic modalities based on Del Brutto’s criteria for accurate diagnosis of NCC. Hence, a combination of serological examination, radiographic report, and clinical data is indispensable. Only diagnosis employing such a comprehensive battery, would uncover the actual burden of NCC. This warrants for development of more valid, specific and affordable diagnostic tools to have better insights of this neglected disease, aiding in estimation of the actual morbidity and mortality around the world.