Analysis of Isocitrate Dehydrogenase -2 (IDH-2) Activity in Human Serum as a Biomarker in Chemotherapy Patients of Breast Carcinoma: A Case-Control Study
Roshni Gavel1, S.P. Mishra2, Seema Khanna3, Rahul Khanna4, Agni Gautam Shah5
1 Postgraduate Resident, Department of Biochemistry, Institute of Medical Sciences, Banaras Hindu University, Varanasi, Uttar Pradesh, India.
2 Associate Professor, Department of Biochemistry, Institute of Medical Sciences, Banaras Hindu University, Varanasi, Uttar Pradesh, India.
3 Associate Professor, Department of General Surgery, Institute of Medical Sciences, Banaras Hindu University, Varanasi, Uttar Pradesh, India.
4 Professor, Department of General Surgery, Institute of Medical Sciences, Banaras Hindu University, Varanasi, Uttar Pradesh, India.
5 Postgraduate Resident, Department of Biochemistry, Institute of Medical Sciences, Banaras Hindu University, Varanasi, Uttar Pradesh, India.
NAME, ADDRESS, E-MAIL ID OF THE CORRESPONDING AUTHOR: Dr. SP Mishra, Associate Professor, Department of Biochemistry, Institute of Medical Sciences, Banaras Hindu University, Varanasi-221005, Uttar Pradesh, India.
E-mail: drsurendram2@gmail.com
Introduction
Breast cancer represents a major public health problem in women worldwide. For many cancers, serum tumour markers play an important role in patient treatment and monitoring. Isocitrate dehydrogenase enzyme is also used as a biomarker for various types of cancer.
Aim
The purpose of this study was to determine serum Isocitrate dehydrogenase-2 (IDH-2) enzyme activity in breast cancer patients (pre and post chemotherapy) and also correlate the changes in enzyme activity with stages of cancer and control groups.
Materials and Methods
In this case-control study, histologically confirmed 40 female patients aged 28-80 years who fulfilled the criteria for diagnosis of invasive breast cancer were selected in our study groups from surgery outpatient department of SS Hospital, BHU, Varanasi, India, and 40 healthy age matched females were selected between October 2013 to July 2015. The estimation of serum IDH-2 enzyme activity in before and after two cycles of neoadjuvant chemotherapy patients was performed by spectrophotometry assay.
Results
The mean serum IDH-2 activity in cases (Mean±SD) was significantly more than control group (p<0.001). The mean serum IDH-2 activity in cases was significantly decrease after neo-adjuvant chemotherapy (p=0.019). In stage II pre chemotherapy patients serum IDH-2 activity was higher than post chemotherapy (p<0.05), but in stage III the correlation between pre and post chemotherapy patients serum IDH-2 activity was not significant (p-value>0.05).
Conclusion
The serum IDH-2 can be a potential biomarker in breast carcinoma and can be used for prognosis and monitoring the chemotherapy response of the patients.
Enzyme activity, Serum biomarker, Neoadjuvant, Spectrophotometry
Introduction
Breast cancer represents a major health problem in women worldwide; with more than one million new cases per annum, it is the most common cancer type and the second leading cause of death from cancer in women [1]. Early detection in order to improve breast cancer outcome and survival remains the cornerstone of breast cancer control. Serum tumour markers play an important role in cancer patient prognosis and diagnosis [2-6]. The role of serum markers in breast tumour is not established. The most common serum markers in breast cancer are CA 15-3 and Carcinoembryonic Antigen (CEA). The recently used markers are breast cancer associated antigen-BR 27.29, Tissue Polypeptide Antigen (TPA), Tissue Polypeptide Specific antigen (TPS) and HER-2 [7-9]. The use of serum marker in breast cancer is aiding early diagnosis, prognosis, response to therapies and monitoring therapy in advanced disease patients. The first identification of a cancer associated Isocitrate Dehydrogenase (IDH) enzyme mutation was in patient with colorectal cancer. Till date, IDH activities and its alteration have been reported in various human cancers [10].
IDH enzyme is a mitochondrial as well as cytoplasmic enzyme, requires Mg2+ or Mn2+ ion as a part of TCA cycle, which catalyzes oxidative decarboxylation of isocitrate to form α-ketoglutarate [11]. Mn2+ in the active site of enzyme interacts with the carbonyl group of the intermediate oxalosuccinate and also stabilizes the enol formed transiently by decarboxylation [12].
The various isoenzymes of IDH are formed in all eukaryotic cells. The mitochondria uses only NAD+ and two other isoforms uses only NADP+ located in mitochondria and cytosol both. IDH encode by 3 types of IDH gene in three polymorphic forms- IDH-1, 2 and 3. IDH1 and IDH2 encode cytosolic and mitochondrial matrix NADP+ dependent (or NADPH dependent) enzymes both are differ from IDH3. [Table/Fig-1] showing that IDH1 and IDH2 catalyze reversible reactions and have no known allosteric modifiers, whereas the reaction catalyzed by IDH3 is irreversible and allosterically regulated by a variety of positive calcium, ADP, citrate, ATP, NADH, and NADPH effectors [13]. The wild type IDH-1/2 converts isocitrate to α-ketoglutarate, producing NADPH in this process. Mutant IDH -1/2 converts α-ketoglutarate to D -2 hydroxygluterate, consuming NADPH. Altered NADPH/NADP+ ratio causes change in redox regulation [14].
Isocitrate dehydrogenase enzymatic activity and molecular mechanism [13].
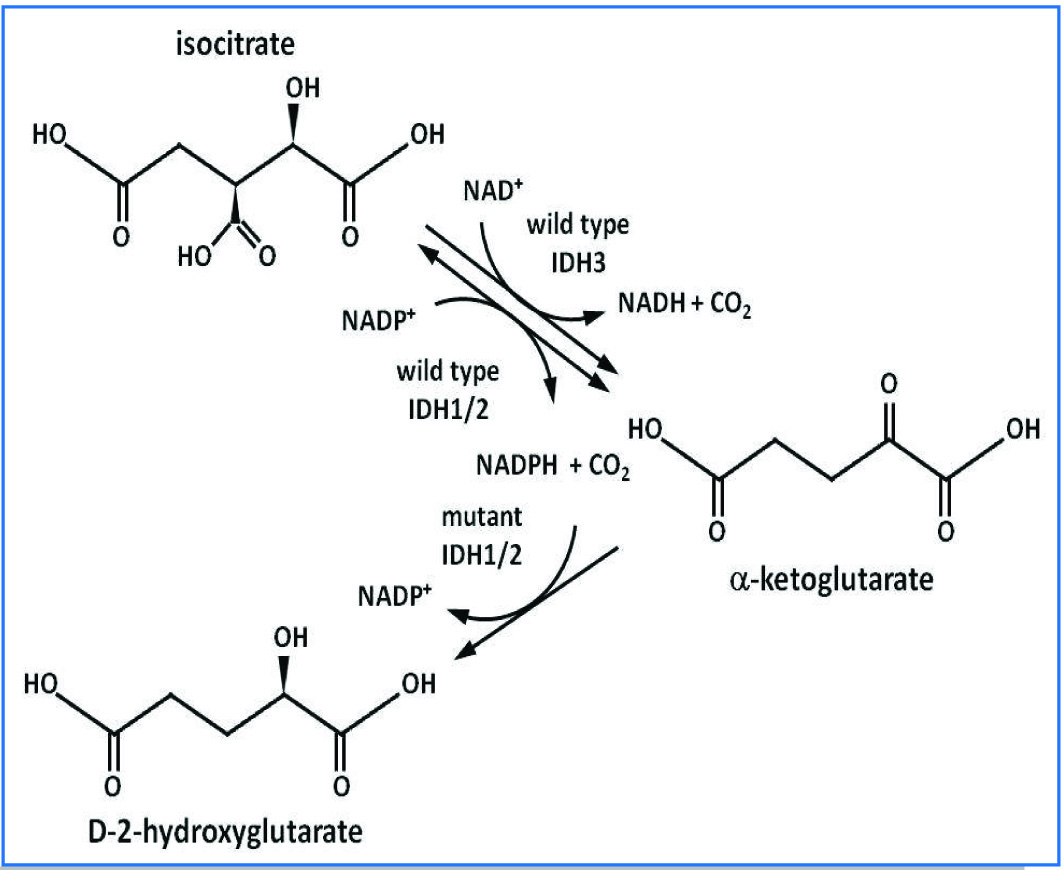
The two NADP dependent forms are located either in mitochondria (IDH-2) or cytoplasm (IDH1), its role in the TCA and in cellular defense against oxidative damage as a source of NADPH2 in HMP shunt. The IDH also plays role in metabolic function like oxidation of polyunsaturated fatty acid within peroxisomes (IDH1) and mitochondria (IDH2) by using the NADP and NADPH generated by their enzymatic activity [15]. The IDH1 is regulated by sterol regulatory element-binding proteins and also generates NADPH for peroxisomal lipogenesis [16]. The IDH1 plays an important role in pancreatic islet cells by increasing insulin secretion [17]. IDH1 and IDH2 play a central role in lipid synthesis and carbohydrate utilization. IDH catalyzes large negative free energy change, it is one of the irreversible reactions in the TCA cycle, and therefore must be carefully regulated to avoid unnecessary depletion of isocitrate [18]. IDH is allosterically regulated by ADP, ATP, NADPH, or NADH [11]. In eukaryotic cells, the activity of IDH-2 increases in response to a variety of oxidative damage [19,20]. Though, the pentose phosphate pathway is the main source of NADPH2, IDH1 and IDH2 enzymes are also important. Further, evidences do exist for their role in cell protection from different insults. IDH1 and IDH2 deficiency leads to increased lipid per oxidation, oxidative DNA damage, intracellular peroxide generation and decreased survival of a fibroblast cell line after exposure to oxidative agents [19].
Only IDH1 and IDH2 gene mutations have been shown to be important in malignancies. Specific mutations in the IDH gene IDH1 have been found in several brain tumours including astrocytomas, oligodendroglioma and glioblastoma multiformis [21]. The NADP+ dependent IDH genes IDH1 and IDH2 are mutated in >75% of low grade gliomas and secondary Glioblastomultiforme (GBM) and 20% of acute myeloid leukaemia (AML) [22]. IDH1 mutation has rapidly emerged as a reliable diagnostic and prognostic marker for identifying low grade gliomas and for distinguishing secondary and primary GBM [23]. Furthermore, in Acute Myeloid Leukaemia (AML) IDH1 and IDH2 mutation were found in up to 20% [24]. These mutations are known to produce 2-hydroxyglutarate from alpha-ketoglutarate. The 2-hydroxyglutarate accumulates to very high concentrations, which inhibits the function of enzymes that are dependent on alpha-ketoglutarate. This leads to a hypermethylated state of DNA and histones, which results in different gene expression that can activate oncogenes and inactivate tumour suppressor genes. Ultimately, this may lead to the cancer.
The causal link between metabolic alterations and cancer formation was revealed only this decade. Recently, mutations in nuclear genes encoding mitochondrial enzymes (Fumarate, Hydratase and Succinate, Dehydrogenase) have been implicated in cancer susceptibility. Over the past year two of the three isoform of the metabolic enzyme IDH (IDH1 and IDH2), were found mutated in high proportions in gliomas [25]. Till now, there have been no studies on this subject in India and data regarding this is lacking. Thus, the study aimed to observe the serum IDH-2 activities in breast cancer patients (pre and post chemotherapy) and also correlate the changes in enzyme activity with stages of cancer.
Materials and Methods
Selection of Patients
This prospective case control study was carried out in the Department of Biochemistry with collaboration of Department of Surgery, Institute of Medical Sciences, Banaras Hindu University, Varanasi, between October 2013 to July 2015. The level of significance is 5% and power of study is 90% of our variates. We selected the study population by the following mathematical formula:
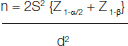
Where the S= Pooled SD
n = no. of cases required for each of the two group d = Difference between the two mean
Z = Standard normal variates
By this formula, we calculated the study population n= 38, but 5% difference in our study population, so the study population (according to 1:1 study) we selected 40 cases and 40 controls. The patients previously untreated but confirmed by histology as invasive breast carcinoma, admitted to General Surgery, Department of Sir Sunderlal Hospital, Varanasi, were taken as cases and 40 normal female patients of same age group and free of disease were taken as controls. Blood samples were collected, before and after two cycles of neo-adjuvant chemotherapy. Ethical clearance was taken from Institute Ethical Committee (IEC, IMS, and BHU) before beginning the study and informed consent was taken before collection of blood sample in every case. After proper history and examination patients underwent fine needle aspiration cytology for confirmation of diagnosis. Patient details were recorded in performa. In this study, we excluded pregnant women, women on oral contraceptive pills for last three months, patients receiving HRT and patients with inflammatory breast diseases.
Sample Collection
Under aseptic precaution 5 ml of venous blood sample was drawn from contra lateral hand of diseased breast cancer patients. The blood thus collected in clean and dry disposable tubes were allowed to stand for 30 minutes at room temperature for the retraction of clot then centrifuged at 3000 rpm for 10 minutes to separate the serum. The serum samples were stored at -20°C in the refrigerator for analysis.
Procedure
The IDH enzyme activity assay kit (Catalog no.-K756-100) provides a simple and direct procedure for IDH activity. It was determined by using isocitrate as the substrate in an enzyme reaction, which resulted in a spectrophotometry (450 nm) product proportional to the enzymatic activity present. One unit of IDH is the amount of enzyme that generated 1.0 mmole of NADPH per minute at pH 8.0 at 37°C.
Calculation
The NADPH standard curve was plotted by taking the end point OD of the standard sample. NADPH generated by the IDH of the samples was obtained from ΔOD (i.e., A1 - A0) of the samples by extra plotting the standard NADPH curve. [Table/Fig-2] shows the standard NADPH curve of our study.
Serum IDH-2 enzyme activity assay standard curve.
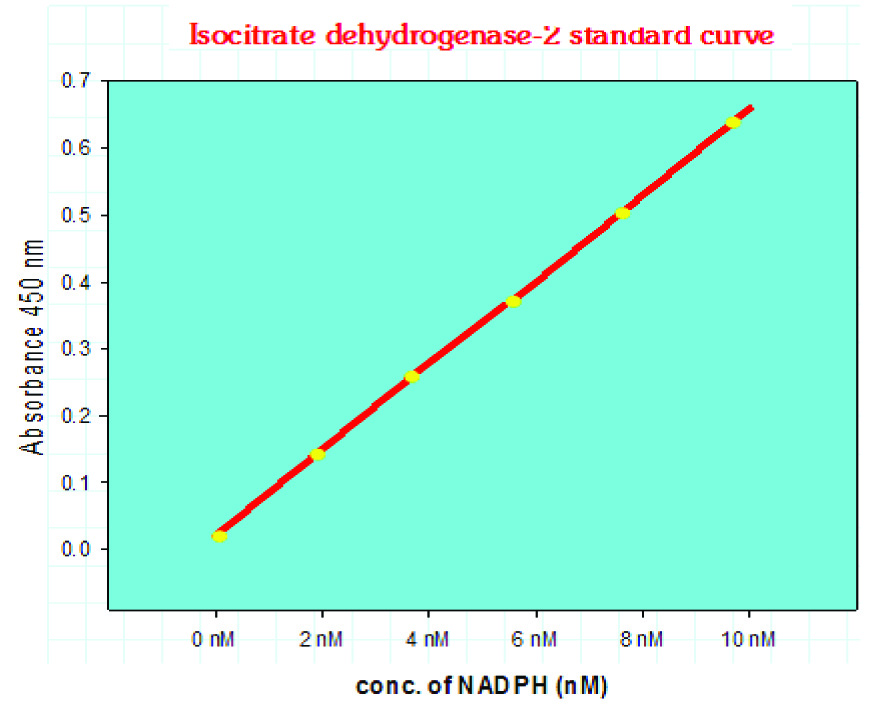
Sample Dilution Factor = nmol/min/ml = mU/ml
Now,
IDH Activity = B/ΔT × V
Where:
B : the NADPH amount from standard curve (in nmol).
T : the reaction time (in min) T1-T0
V : the sample volume added into the reaction well (in ml).
Statistical Analysis
All statistical analysis was done by using trial version-16.0, SPSS software. Differences between unrelated groups were assessed by using student’s independent t–test and differences between pre and post chemo groups were assessed by using paired student’s t-test. The data were expressed as mean± SD, p-value<0.05 is considered as significant and mean± SD, p-value<0.01 is highly significant.
Results
The observations regarding the estimation of serum IDH-2 were done at the time of presentation and after two cycles of neo-adjuvant chemotherapy.
The changes in the level of Serum IDH-2 following two cycles of chemotherapy have been correlated with staging of tumour and prognosis after chemotherapy in the tumour patients. The mean serum IDH-2 activity in carcinoma breast patients was 0.74±0.44 mU/ml and in control group was 0.44±0.30 mU/ml (p<0.05). There was an increase in activity of serum IDH-2 seen in cases as compared to control [Table/Fig-3]. Also, compared pre chemotherapy serum IDH-2 activity (mean=0.74±0.44 mU/ml) with post chemotherapy serum IDH-2 activity (mean=0.46±0.30 mU/ml). Overall there was a higher activity of serum IDH-2 seen in pre chemotherapy patients (p= 0.019). Both the results were significant [Table/Fig-4].
Comparison of serum IDH-2 enzyme activity between breast cancer patients (pre chemotherapy) and controls.
| Variables | IDH-2 Activity(mU/ml) (Mean ± SD) | t- value | p-value |
|---|
| Cases (n=40) | 0.74±0.44 | 2.532 | 0.007 |
| Controls (n=40) | 0.44±0.30 |
*by unpaired t-test.
Comparison of serum IDH-2 enzyme activity between breast cancer patients of pre chemotherapy and post chemotherapy.
| variables | IDH-2 activity (mu/ml) (Mean ± SD) | t- value | p-value |
|---|
| Pre chemotherapy (n=40) | 0.74 ± 0.44 | 2.446 | 0.0190 |
| Post chemotherapy (n=40) | 0.46 ± 0.30 |
* by paired t-test.
We also compared breast carcinoma stage II pre chemotherapy patients’ serum IDH-2 activity (mean 0.93±0.55 mU/ml) was higher as compared to post chemotherapy patients (mean 0.63±0.32 mU/ml) (p-value< 0.05). But in stage III breast carcinoma serum IDH-2 activity was not significant (p-value > 0.05) [Table/Fig-5].
Comparison of pre and post chemotherapy Serum IDH-2 activity with tumour stage.
| Stage | Pre chemo IDH-2 activity (Mean ± SD) | Post chemo IDH-2 activity (Mean ± SD) | t-value | p-value |
|---|
| I (n=0) | - | - | - | - |
| II (n=17) | 0.93 ± 0.55 | 0.63 ± 0.32 | 1.907 | 0.033 |
| III(n=23) | 0.63 ± 0.26 | 0.57 ± 0.28 | 0.782 | 0.219 |
| IV (n=0) | - | - | - | - |
Discussion
Breast cancer is most frequently diagnosed cancer and the leading causes of death among women worldwide [1]. Detection and identification of prognostic markers for predicting therapeutic response can be useful for breast cancer treatment. With increasing range of cancer therapies, the clinician must receive guidance as to which patients should be treated with which drug and the patient is responding to therapy or not. Biomarkers of breast cancer are necessary for prognosis, follow up and prediction of treatment. It has a very varied clinical, pathologic, molecular features and treatment modalities [26]. Prognostic biomarkers provide information regarding outcome irrespective of therapy, while predictive biomarkers provide information regarding response to therapy [27].
In cancer cells dysregulation of metabolism is a common phenomenon. Normally, in the liver cells (hepatocytes) IDH1 is most highly expressed but IDH2 is highly expressed in muscles [28]. The sub cellular distribution of NADP+ dependent IDH activity is differed in different tissue level. The majority of enzyme activity in ovary, mammary gland, and liver are located in the cytosol, whereas in heart and skeletal muscle most activity is located in the mitochondria [29]. The activity of cytosolic NADP+ dependent IDH increases markedly during hormone-induced development of the immature ovary and in the mammary gland the onset of lactation [28,29]. The reactions catalyzed by IDH1/2 are reversible while the similar reaction catalyzed by IDH3 is irreversible because only the gene mutations of IDH1 and IDH2 have been shown to be important in malignancies.
IDH1 and IDH2 play important role in antioxidant deficiency leads to increased lipid per oxidation, oxidative DNA damage; intracellular peroxide generation [20]. These mutations are known to produce 2-hydroxyglutarate from alpha ketoglutarate. A 2-hydroxyglutarate accumulates to very high concentrations, which inhibits the function of enzymes that are dependent on alpha ketoglutarate. This leads to a hypermethylated state of DNA and histones, which results in different gene expression that can activate oncogenes and inactivate tumour suppressor genes, ultimately this may lead to cancer. The inference of these findings is that patients with decrease of serum IDH-2 enzyme activity following chemotherapy are responding to their chemotherapy protocol. Thus, IDH-2 enzyme activity in serum may be a useful predictor of response to chemotherapy. The lacunae of our study are that it requires long term monitoring after treatment and genomic study also required.
Conclusion
Analysis of IDH-2 enzyme may be used as a biomarker in breast cancer patient and strongly suggest that it can be used to determine the efficiency of cytotoxic drug treatment and monitoring the chemotherapy drug response in patient serum samples. The ability to compare the efficiencies of different treatment modalities would be very useful during development of new anticancer drugs.
*by unpaired t-test.
* by paired t-test.
[1]. Jemal A, Siegel R, Ward E, Murray T, Xu J, Thun MJ, Cancer statistics 2007CA: a Cancer Journal for Clinicians 2007 57(1):43-66. [Google Scholar]
[2]. Bosl GJ, Motzer RJ, Testicular germ-cell cancerNew England Journal of Medicine 1997 337(4):242-54. [Google Scholar]
[3]. Fisher PM, Hancock BW, Gestational trophoblastic diseases and their treatmentCancer Treatment Reviews 1997 23(1):1-6. [Google Scholar]
[4]. Rustin GJ, Use of CA-125 to assess response to new agents in ovarian cancer trialsJournal of Clinical Oncology 2003 21(10 suppl):187s-93s. [Google Scholar]
[5]. Duffy MJ, Carcinoembryonic antigen as a marker for colorectal cancer: is it clinically useful?Clinical Chemistry 2001 47(4):624-30. [Google Scholar]
[6]. Parker C, Active surveillance: towards a new paradigm in the management of early prostate cancerThe Lancet Oncology 2004 5(2):101-06. [Google Scholar]
[7]. Duffy MJ, CA 15-3 and related mucins as circulating markers in breast cancerAnnals of Clinical Biochemistry 1999 36(5):579-86. [Google Scholar]
[8]. Cheung KL, Graves CR, Robertson JF, Tumour marker measurements in the diagnosis and monitoring of breast cancerCancer Treatment Reviews 2000 26(2):91-102. [Google Scholar]
[9]. Nicolini A, Carpi A, Postoperative follow-up of breast cancer patients: overview and progress in the use of tumour markersTumour Biology 2000 21(4):235-48. [Google Scholar]
[10]. Sjöblom T, Jones S, Wood LD, Parsons DW, Lin J, Barber TD, The consensus coding sequences of human breast and colorectal cancersScience 2006 314(5797):268-74. [Google Scholar]
[11]. Lehninger AL, Nelson DL, Cox MM, Lehninger’s Principles of BiochemistryWH Freeman 2005 :609-611. [Google Scholar]
[12]. Garrett R, Grisham CM, Biochemistry 2008 BostonCengage Learning Inc:621 [Google Scholar]
[13]. Mellai M, Schiffer D, Annovazzi L, Caldera V, The distribution and significance of IDH mutations in gliomas 2013 INTECH Open Access Publisher [Google Scholar]
[14]. Gálvez S, Gadal P, On the function of the NADP-dependent isocitrate dehydrogenase isoenzymes in living organismsPlant Science 1995 105(1):1-4. [Google Scholar]
[15]. Minard KI, McAlister-Henn L, Dependence of peroxisomal β-oxidation on cytosolic sources of NADPHJournal of Biological Chemistry 1999 274(6):3402-06. [Google Scholar]
[16]. Shechter I, Dai P, Huo L, Guan G, IDH1 gene transcription is sterol regulated and activated by SREBP-1a and SREBP-2 in human hepatoma HepG2 cells evidence that IDH1 may regulate lipogenesis in hepatic cellsJournal of Lipid Research 2003 44(11):2169-80. [Google Scholar]
[17]. Ronnebaum SM, Ilkayeva O, Burgess SC, Joseph JW, Lu D, Stevens RD, A pyruvate cycling pathway involving cytosolic NADP-dependent isocitrate dehydrogenase regulates glucose-stimulated insulin secretionJournal of Biological Chemistry 2006 281(41):30593-602. [Google Scholar]
[18]. Kwon SJ, Park JW, Choi WK, Kim IH, Kim KW, Inhibition of metal-catalyzed oxidation systems by a yeast protector protein in the presence of thioredoxinBiochemical and Biophysical Research Communications 1994 201(1):8-15. [Google Scholar]
[19]. Lee SM, Koh HJ, Park DC, Song BJ, Huh TL, Park JW, Cytosolic NADP+-dependent isocitrate dehydrogenase status modulates oxidative damage to cellsFree Radical Biology and Medicine 2002 32(11):1185-96. [Google Scholar]
[20]. Nakamura H, Thioredoxin and its related molecules: update 2005Antioxidants & Redox Signaling 2005 7(5-6):823-28. [Google Scholar]
[21]. Bleeker FE, Molenaar RJ, Leenstra S, Recent advances in the molecular understanding of glioblastomaJournal of Neuro-oncology 2012 108(1):11-27. [Google Scholar]
[22]. Mardis ER, Ding L, Dooling DJ, Larson DE, McLellan MD, Chen K, Recurring mutations found by sequencing an acute myeloid leukaemia genomeNew England Journal of Medicine 2009 361(11):1058-66. [Google Scholar]
[23]. Ducray F, El Hallani S, Idbaih A, Diagnostic and prognostic markers in gliomasCurrent Opinion in Oncology 2009 21(6):537-42. [Google Scholar]
[24]. Ward PS, Patel J, Wise DR, Abdel-Wahab O, Bennett BD, Coller HA, The common feature of leukaemia-associated IDH1 and IDH2 mutations is a neomorphic enzyme activity converting α-ketoglutarate to 2-hydroxyglutarateCancer Cell 2010 17(3):225-34. [Google Scholar]
[25]. Parsons DW, Jones S, Zhang X, Lin JC, Leary RJ, Angenendt P, An integrated genomic analysis of human glioblastoma multiformeScience 2008 321(5897):1807-12. [Google Scholar]
[26]. Bhatt AN, Mathur R, Farooque A, Verma A, Dwarakanath BS, Cancer biomarkers-current perspectivesIndian Journal of Medical Research 2010 132(2):129 [Google Scholar]
[27]. Oldenhuis CN, Oosting SF, Gietema JA, DeVries EG, Prognostic versus predictive value of biomarkers in oncologyEuropean Journal of Cancer 2008 44(7):946-53. [Google Scholar]
[28]. Haselbeck RJ, McAlister-Henn L, Function and expression of yeast mitochondrial NAD-and NADP-specific isocitrate dehydrogenasesJournal of Biological Chemistry 1993 268(16):12116-22. [Google Scholar]
[29]. Flint AP, Denton RM, The role of nicotinamide–adenine dinucleotide phosphate-dependent malate dehydrogenase and isocitrate dehydrogenase in the supply of reduced nicotinamide–adenine dinucleotide phosphate for steroidogenesis in the superovulated rat ovaryBiochemical Journal 1970 117(1):73-83. [Google Scholar]