Angiogenesis is the process by which new blood vessels are produced by pre-existing vasculature [1]. The importance of vascular networks in the development and maintenance of tissues have been demonstrated in many physiologic and pathologic processes, such as embryogenesis, wound healing, inflammation and tumour progression [2]. Angiogenesis requires a combination of growth factors and pro-angiogenic cytokines (VEGF) and is regulated by an equally varied group of inhibitors (angiostatin) of neovascularization. An increase in the level of angiogenic stimuli and a simultaneous decrease in the level of angiogenic inhibitors produced by tumour cells, stromal cells and inflammatory cells such as mast cells and macrophages initiates angiogenesis [3].
VEGF is not only a potent mitogenic agent for endothelial cell proliferation but also plays an important role in the pathogenesis of reactive, vascular proliferative lesions like PG and reparative, chronic inflammatory lesions like PAG. To our knowledge there are no studies regarding the analysis of expression of VEGF in the PG and PAG. The measurement of its role may be important for better understanding of the pathogenesis of neoproliferative lesions. As inflammation and VEGF expression are seen in both PG and PAG, the present study was aimed to evaluate, compare and correlate the expression of VEGF along with inflammation in both the lesions.
Materials and Methods
The present retrospective study was carried out in the Department of Oral and Maxillofacial Pathology, St Joseph Dental College, Eluru, Andhra Pradesh, India during year 2014 to 2015. Paraffin embedded tissue blocks of histologically diagnosed cases of PG and PAG were retrieved, 20 each from the archives.
Early phase PG occurring on gingiva in relation to plaque and calculus were only included in the study. As hormones and drugs alter angiogenesis, thereby affecting VEGF expression, PG occurring during pregnancy and drug induced were excluded. PG and PAG were considered as two comparative groups because both the lesions have similar histopathological features, still PG has extensive growth when compared to restricted growth of PAG.
The cases were selected randomly and the tissue blocks of all 40 samples of 4 μm thickness were sectioned and were subjected to conventional H&E staining and Immunohistochemistry (IHC) using VEGF marker (Biogenics). Then, they were analysed under Trinocular Olympus Bx53Progres CT research microscope and the expression of VEGF marker was evaluated by using Mean Vascular Count (MVC) or vascular index and inflammation by Morphological Index (MI).
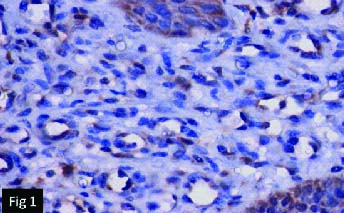
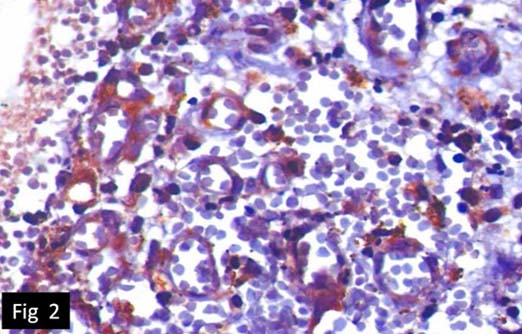
Mean Vascular Count (MVC) Index: After immunohistochemical (IHC) staining, the tissue sections were examined at 40X magnification under light microscopy. The expression of VEGF was evaluated in the connective tissue of both PG and PAG. Ten fields from each sample showing highest vascularization were identified subjectively. In these areas, numbers of positive vessels were counted and results were tabulated [Table/Fig-1,2].
VEGF expression in pyogenic granuloma. (IHC) (40X).
VEGF expression in periapical granuloma. (IHC) (40X).
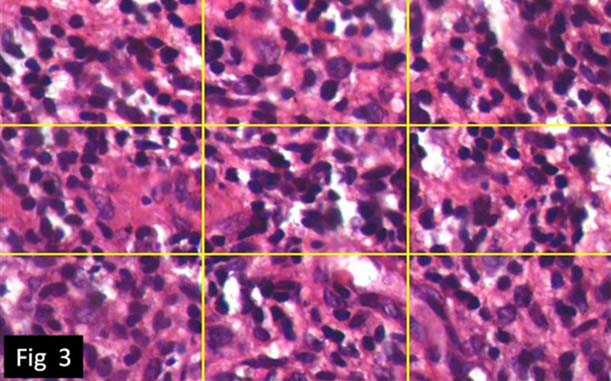
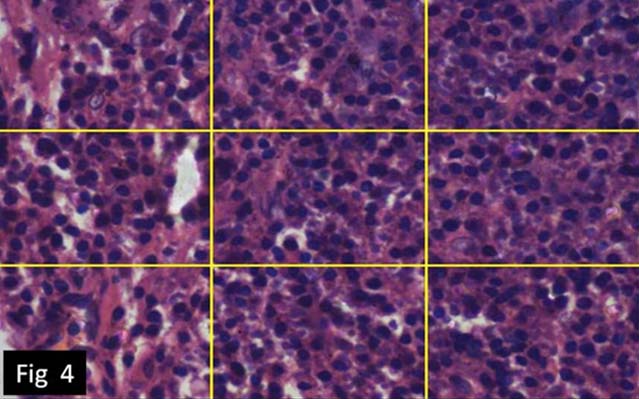
Morphological Index (MI): After the conventional H&E staining, the tissue sections were examined for inflammatory index at 40X magnification and three fields from each slide showing highest inflammation were identified subjectively. Each field was divided into nine quadrants and examined for inflammatory cells. Any quadrants with atleast five inflammatory cells were considered as positive for inflammation and quadrant with less than five inflammatory cells was considered as negative for inflammation. Positive quadrants were numbered as 1 and negative quadrants as 0. Further mean of nine quadrants was tabulated and analysed statistically. [Table/Fig-3,4].
Inflammatory infiltrate in pyogenic granuloma (H&E) for morphologic index. (40X).
Inflammatory infiltrate in periapical granuloma (H&E) for morphologic index. (40X).
Statistical Analysis
Unpaired t-test was done to compare the expression of VEGF (MVC) index in PG and PAG, Mann-Whitney U test was done to evaluate the MI in PG and PAG and Spearman correlation coefficient was done to evaluate the correlation between inflammation and VEGF expression in PG and PAG.
Results
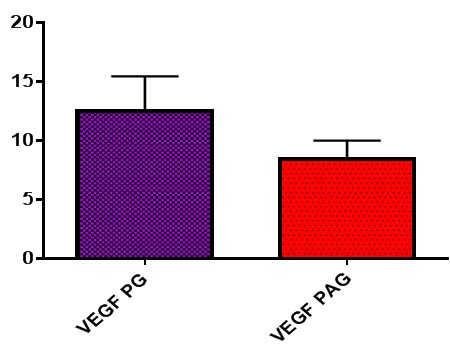
For MVC index, total numbers of positive vessels in 10 fields were counted and mean was taken. The MVC index was 12.47±0.66 in PG, comparatively more than PAG which was about 8.440±0.34. The difference in mean was statistically significant (p<0.001) [Table/Fig-5] by using unpaired t-test.
Comparison of VEGF expression in pyogenic granuloma and periapical granuloma by using unpaired t-test.
VEGF PG-Vascular endothelial growth factor in pyogenic granuloma, VEGF PAG- Vascular endothelial growth factor in periapical granuloma
For MI index, quadrants with atleast five inflammatory cells were considered as positive and scored as 1. Quadrants less than 5 inflammatory cells were considered as negative and scored as 0. Mean of 9 quadrants were taken. The mean morphological index of inflammation was 8.48±0.98 in PG and 8.91±0.12 in PAG. So difference in inflammation between PG and PAG was statistically insignificant (p=0.070) [Table/Fig-6] by using Mann-Whitney U test.
Comparison of inflammation in pyogenic granuloma and periapical granuloma by using Mann-Whitney U test.
INF PG-Inflammation in pyogenic granuloma, INF PAG- Inflammation in periapical granuloma

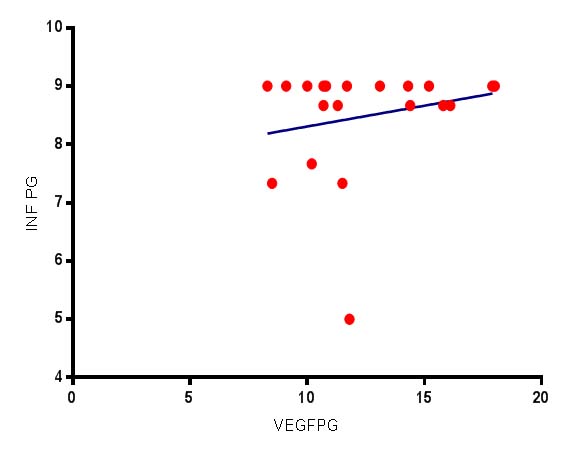
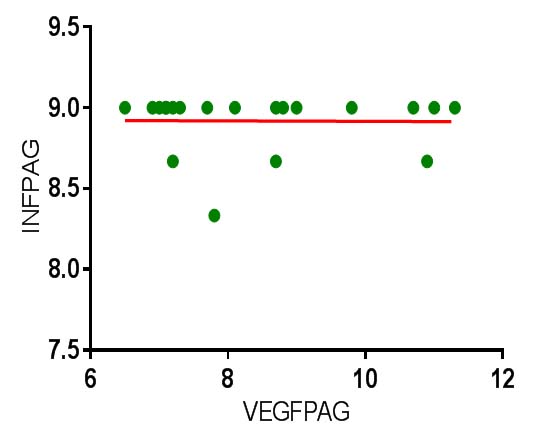
The correlation between inflammation and expression of VEGF in PG showed very weak positive correlation which was statistically insignificant (R=0.07980) (p=0.7381) [Table/Fig-7] by using Spearman correlation coefficient-test. The correlation between inflammation and expression of VEGF in PAG showed very weak negative correlation which was statistically insignificant (R=-0.1004) (p=0.6735) [Table/Fig-8] by using Spearman correlation coefficient-test. PG showed more expression of VEGF when compared to PAG with no significant difference in inflammation between PG and PAG. In PG, VEGF expression and inflammation showed weak positive correlation whereas in PAG, showed weak negative correlation.
Correlation of inflammation and VEGF expression in pyogenic granuloma and periapical granuloma by using spearman correlation coefficient-test.
INF PG-Inflammation in pyogenic granuloma, VEGF PG-Vascular endothelial growth factor in pyogenic granuloma.
Correlation of inflammation and VEGF expression in periapical granuloma by using spearman correlation coefficient-test.
INF PAG- Inflammation in periapical granuloma, VEGF PAG- Vascular endothelial growth factor in periapical granuloma
Discussion
Angiogenesis is mediated through the interplay of factors such as angiogenic stimuli and angiogenic inhibitors. These factors are produced by tumor cells, stromal cells and inflammatory cells such as mast cells and macrophages. It was suggested by “balance hypothesis” that changes in the relative balance of activators and inhibitors of angiogenesis can turn the angiogenic switch “on” or “off” and lead to vessel formation or regression [4]. Alterations in this regulatory process may lead to insufficient or excessive angiogenesis which probably results in vascular reactive lesions like PG. VEGF is an extremely potent and specific angiogenic factor with a selective mitogenic effect on vascular endothelial cells. Therefore, has been implicated as an important factor in PAG development.
VEGF expression in PG: In the present study, PG showed significant expression of VEGF immunopositive vessels. The possible reasons for this could be presence of prominent capillary growth in hyperplastic granulation tissue, suggestive of strong activity of angiogenesis. In this regard, PG may be considered as an exuberant expression of granulation tissue formation in prolonged healing processes. As the lesion progresses to late phases of pathogenesis, the budding capillaries mature to form well organized mature vessels and VEGF expression decreases. This is probably due to regulation of VEGF expression by angiogenesis inhibitors. In the present study, higher expression of VEGF was seen in PG because most of the cases included in the study were in early phases of lesion. On the other hand, locally increased concentrations of reactive oxygen species strongly stimulate VEGF production by endothelial cells. Therefore, the existence of local inflammatory cytokines might explain the induction of VEGF [5]. The other factors that regulate angiogenesis in PG are angiogenesis enhancers such as bFGF, Estrogen Receptors (ER), Thrombospondin-1 (TSP-1) and inhibitors such as angiostatin [4].
The major source for VEGF is macrophages and surface epithelial cells, especially during chronic inflammatory and infectious conditions [6]. Though we have not considered the epithelial expression of VEGF in the present study, considerable amount of overlap was seen between the epithelium and connective tissue expression especially at the papillary portion of the stroma. Similar expression of VEGF was seen in periapical lesions which have bioactivity to increase vascular permeability, relating partially to the accumulation of inflammatory cells and cyst fluid. Therefore, several biological functions are described for VEGF, such as inducing microvascular permeability and a potent mitogenic factor for endothelial cells. Leonardi R et al., suggested that inflammatory cells and fibroblasts could be responsible for synthesis of VEGF in the early phases of periapical lesion development and epithelial cells in later stages. Recent evidence suggests that capillary regression can also be mediated by macrophages, since they produce substances which are inhibitory to endothelial cell growth [7].
Inflammation in PAG and PG: Inflammation in PAG and PG showed no significant difference, as both the lesions are reactive and reparative response to the trauma and bacterial toxins respectively. In PAG, the inflammatory changes have no specific features. According to the severity of infection the inflammatory infiltrate can be predominantly polymorphonuclear cells or monocytes and plasma cells. Initially the inflammation is confined to the minute space between the apex and its surrounding bone, but resorption of the latter makes a space in chronic lesions becomes a rounded cavity to accommodate the proliferating granulation tissue, thereby forming an apical “granuloma” [8]. PG is a reactive hyperplasia in which inflammation plays an important role in the pathogenesis. Increased amount of inflammation represents an exuberant tissue response to local irritation or trauma. Due to the irritation from the poor oral hygiene, dental plaque and calculus or overhanging restorations, gingival tissue results in synchronized activation of host defense mechanism provoking the inflammation, mediated by the several chemokines and subsequent expression of the VEGF induced angiogenesis as a reactive response to the underlying irritation.
The type of the immune response in the PAG tissue is determined by their apically resident bacteria and continuous exposure of periapical tissues to the bacterial toxins which evoke inflammation and subsequent expression of several growth factors results in the new capillaries formation for rapidly proliferating granulation tissue [9]. Thus, the inflammatory mediators play an important role in the angiogenesis and subsequent pathogenesis of both PG and PAG.
In the present study, though there is no significant difference in inflammation between PG and PAG but significant difference was seen in expression of VEGF among them, indicating a marked difference at molecular level. There may be other factors that down regulate the expression of angiogenic mediators in PAG even though there is inflammation to a larger extent. In PG, VEGF expression and inflammation showed positive correlation. The local inflammatory cytokines (interleukin-1, TNF-α) induce and upregulate the expression of VEGF, and also locally increased concentration of Reactive Oxygen Species (ROS) strongly stimulate VEGF production by endothelial cells. Other possible reasons could be organization phase of the lesion. The vascularity and inflammation is reduced when the lesion shows maturation. During maturation, vascularity is reduced due to complete organization into mature vessels and the inflammatory component is replaced with the mature fibrous component, hence reducing the expression of VEGF [10]. In the present study, cases included were in early phase, so positive correlation was observed between inflammation and VEGF expression.
VEGF expression and inflammation in PAG showed negative correlation. This difference might be attributed to the morphometric analysis, developmental phase of the lesion, as well as the sample size included in the study. Early stage of PAG has inflammatory oedema with marked infiltration of macrophages, with or without neutrophils and a few lymphocytes. The inflammatory mediators play a central role in PAG formation. As already discussed, the local environment is also one of the factors because PAG is an intraosseous lesion and the availability of blood vessels limited to the budding new capillaries in neovascularization process. Chronic inflammatory cells like lymphocytes and plasma cells dominate with numerous blood vessels in intermediate stage [11]. The late or healing stage shows chronic inflammatory cells and fewer blood vessels, with fibroblasts. During the cystic transformation stage, there is marked reduction in vascularity and nutrient supply resulting in the degeneration of central cells in the granuloma and show cystic transformation. It could be hypothesized that the decrease in the number of blood vessels might be related to a possible conversion of granulation tissue to a cyst. It could contemplate that, though the amount of inflammation remained unchanged, the expression of VEGF decreases, during the healing stage and cystic transformation stage of PAG. Theoretically, we divide the PAG formation into various stages. But the patient approaches the dentist only when the tooth is symptomatic and by the time biopsy is taken, the patient must have had atleast a course of antibiotic therapy which could have decreased the inflammation component. Moreover, since the lesion is intraosseous and it seldom symptomatic the patient may present at later stage during which the bone resorptive factors come into play and these bone resorptive factors could be inhibitors of VEGF expression.
Other possible reasons might be molecular mechanisms such as methylation and DNA sequence variation. These mechanisms can change angiogenic proteins levels and consequently angiogenesis in cysts and granulomas [12,13]. Thereby in the present study negative correlation between VEGF expression and inflammation was obtained. But, the other studies of Nonaka CF et al., Graziani F et al., have revealed that, there is a positive correlation between VEGF expression and inflammation [7,14]. This difference may be ascribed to the morphometric analysis of inflammatory cells used in the study. Other studies used grading system for inflammation and in the present study; morphometric index of inflammation was intended from the representative area. Differences in representative areas taken for studying inflammation and vascularity are unavoidable. The negative correlation between the inflammation and VEGF is due to the developmental stage of the lesion that was included in the study. Though, there is increased amount of inflammation during the maturation phase of the lesion, the VEGF expression is reduced due to the healing phase or cystic transformation phase by different mechanisms. The cases included in the present study were in the late maturation phase as well as the sample size included in the study also influences the results obtained.
Limitation
In the present study, only histopathological features were assessed. Whereas, clinical parameters and follow up were not considered, so we could not predict the reason for recurrence. Moreover, all the cases were not graded according to the developmental phases. Few of the cases showed higher expression and majority of the cases showed decreased expression because those cases were in late phases. Further studies with larger sample size considering clinical parameters, grading and follow up are required to set the possible mode of therapy for these lesions.
Conclusion
Pyogenic granuloma and periapical granuloma though similar histologically, differ in their clinical presentation, mechanisms of formation and molecular sketch. Thereby raised expression of VEGF marker was established emphasizing the fact that all histologically similar lesions need not have similar clinical course and molecular depiction. The current explanation is that, during normal physiological angiogenesis there is supportive interaction between positive and negative regulatory molecules whereas this interaction gets apparently disrupted in tumors, tumor like lesions and chronic inflammatory diseases. We suggest further studies should be conducted to focus on the interaction between positive and negative regulators. The implications of these findings are crucial for the development of therapeutic strategies for solid tumors and other angiogenesis-dependent disorders.