Carcinoma cervix is the most common cause of cancer related mortality among women in developing nations [1]. Hence, early diagnosis and appropriate management is the key to reduce the disease burden and enhance the quality of life and life expectancy of such women.
MRI has become a corner stone in the evaluation of local extent of cervical carcinoma, assessment of pelvic lymph nodes also plays a vital role in guiding management [2-5]. Post contrast MR imaging following intravenous administration of gadolinium based contrast is widely practiced as part of a more comprehensive imaging package for evaluation of carcinoma cervix. However, studies have doubted if post contrast imaging can add to the information provided by the routine T1 and T2 weighted imaging protocols [6,7].
In view of the above controversy relating to utility of post contrast MRI in evaluation of carcinoma cervix, this study was carried out.
This study was conducted to find the accuracy of T1 and T2 weighted non contrast MR protocol in comparison to post contrast spin echo T1 weighted fat suppressed imaging in evaluating the local extent of the carcinoma cervix.
The study also intends to identify the utility of MR sequences and planes of acquisition in the present context and develop a short and crisp protocol for the detailed evaluation of cervical cancer.
It is also intended to identify the ambiguities where utility of post contrast MRI may add value as a problem solving tool.
Materials and Methods
A prospective study was conducted in the Department of Radiodiagnosis, JJM Medical College Hospital, Davangere, between June 2016 and December 2016. A total of 45 females of age between 32 and 68 years and a mean age of 50.7 years with biopsy proven cervical malignancy were referred for MRI of pelvis for local staging. Sample size was calculated using precession based sample size calculation formula. Institutional Ethical Committee clearance was obtained for this study.
Institutional gynaecologists after detailed examination of the patients, arrived at a FIGO staging (International Federation of Gynaecology and Obstetrics) [8] and recommended MRI to know the detailed extent of the cervical lesion.
After taking informed consent, MRI scan of the pelvis was performed with a 1.5 Tesla MRI system (Philips Achieva). Prior to the scan, 10-15 ml of lignocaine jelly was instilled into the vagina to obtain distension of vagina and fornices [9]. Body coil was used for MRI acquisition. The MRI protocol followed is as per [Table/Fig-1].
MRI imaging protocol followed in this study.
| Parameters | T2 Saggital FS* | T1 Axial | T2 Axial | T2 Axial FS* | T2 Coronal | T1 FS* Axial | T1 FS* – 3 planes post contrast |
|---|
| Repetition Time (TR) in milliseconds | 3714 | 495 | 3657 | 4454 | 5348 | 526 | 526 |
| Time to Echo (TE) in milliseconds | 80 | 10 | 100 | 80 | 90 | 10 | 10 |
| Slice thickness | 4 mm | 4 mm | 4 mm | 4 mm | 4 mm | 4mm | 4mm |
| Number of averages | 3 | 2 | 2 | 3 | 3 | 2 | 2 |
FS – Fat Suppressed.
Initially sagittal T2 weighted images of the pelvis were acquired to identify the plane of the uterus and cervix. Axial and coronal imaging were planned perpendicular and parallel to the plane of cervix. Gadobenate dimeglumine (MULTIHANCE 529mg/ml) was administered intravenously at the dosage of 0.1 mmol/kg body weight and post contrast fat suppressed Spin Echo T1 weighted images were obtained.
Two radiologists with five years of experience each in gynaecological imaging, evaluated the non contrast and contrast enhanced MR images of all patients separately and reported the local extent and staging of the cervical lesions. They were blinded to the clinical staging and also to each other. Interobserver variability and statistical significance were calculated.
On non contrast images interruption of the hypointense cervical stroma on T2 weighted images was considered definitive evidence of parametrial extension [Table/Fig-2]. Similarly interruption of the hypointense wall of the urinary bladder or rectum with the abnormality in continuity with the cervical mass was considered bladder/bowel infiltration. Focal loss of T2 hypointensity or extension of the mass/enhancement into upper two third or lower third vagina were considered accordingly.
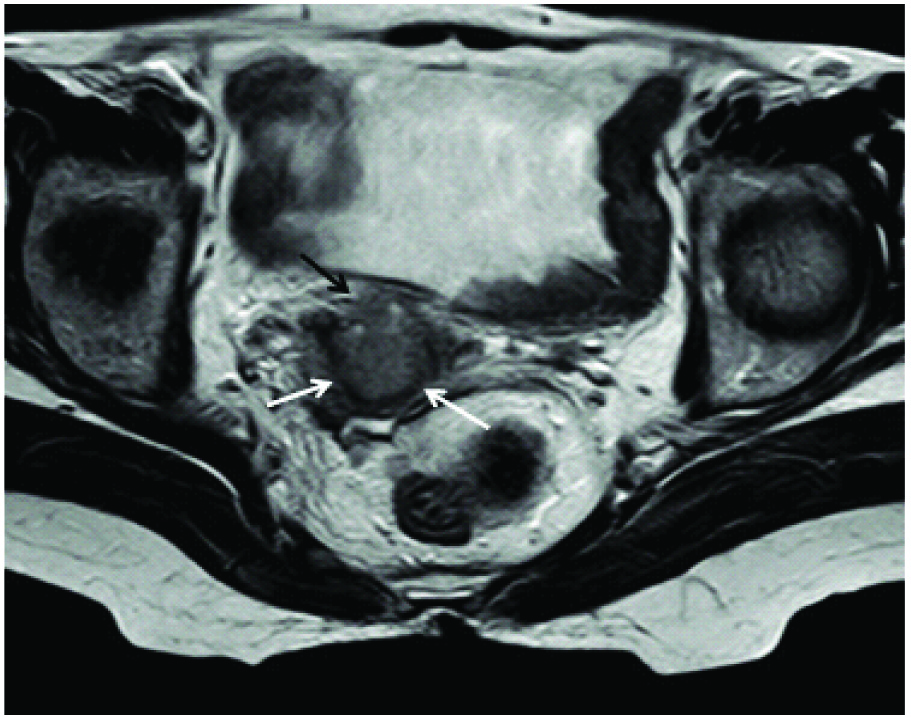
T2 weighted axial image showing an endocervical mass infiltrating into the parametrium anteriorly (black arrow), seen as disruption of the hypointense (white arrows) cervical stroma anteriorly.
Extension of the T2 hyperintensity/enhancement on post contrast scans to less than 3 mm of the lateral pelvic wall (obturator internus, pyriformis, levator ani) or occurrence of hydroureter concomitantly on the ipsilateral side of such extension was considered as lateral pelvic wall invasion.
The extension of the enhancement outside the confines of the cervix was identified as parametrial extension on post contrast imaging. This enhancement could be in the form of spiculated or linear enhancement adjacent to cervix [10,11].
Enhancement of urinary bladder and rectal wall in continuity and synchronised with the cervical lesion was identified as infiltration.
Patients with staging suitable for surgical intervention were operated within two weeks of imaging. Findings on non enhanced MR images were compared with findings on contrast enhanced MR images. Both these findings were then correlated statistically with peroperative findings, wherever available.
Results
Cases were of age between 32 and 68 years with a mean age of 50.7 years. Clinically, of the 45 females, 17 were assessed to have stage 2b disease with parametrial extension. Ten patients were evaluated as having stage 1b disease with vagina and vaginal fornices free. seven patients were found to have extension into upper two thirds of vagina clinically (stage 2a), five cases were suspected to have disease infiltration into urinary bladder (stage 4), four cases showed absence of plane between mass and lateral pelvic wall (stage 3b). Two cases showed extension into lower third of vagina. The breakdown of number of cases as per staging is depicted in [Table/Fig-3].
Breakdown of cases into clinical staging.
| Cases | Clinical staging (FIGO*) [8] |
|---|
| 10 | 1b |
| 07 | 2a |
| 17 | 2b |
| 02 | 3a |
| 04 | 3b |
| 5 | 4 |
FIGO – International Federation of Gynaecology and Obstetrics.
There was 100% agreement (kappa coefficient 1) between the two radiologists in interpreting non contrast scans. There was also 91.1% interobserver agreement (kappa coefficient of 0.9) for post contrast images. There was disagreement among the observers in interpreting post contrast images in four cases, relating to extension into the parametrium. The observer interpretations are depicted in [Table/Fig-4,5].
Interpretations of observer 1.
| FIGO staging | Number of patients |
|---|
| Clinical examination | MRI (non contrast) | MRI – post contrast scan |
|---|
| 1b | 10 | 8 (no extension into parametrium) + 2 (extension into parametrium) | 6 (no extension into parametrium) + 4 (extension into parametrium) |
| 2a | 07 | 5 (extension into upper 2/3 vagina) + 2 (extension into parametrium) | 5 (extension into upper 2/3 vagina) + 2 (extension into parametrium) |
| 2b | 17 | 15 (extension into parametrium only) + 2 (Bladder infiltration) | 15 (extension into parametrium only) + 2 (Bladder infiltration) |
| 3a | 02 | 02 (extension into lower third vagina) | 02 (extension into lower third vagina) |
| 3b | 04 | 04 (extension into pelvic wall) | 04 (extension into pelvic wall) |
| 4 | 5 | 04 (Bladder infiltration) + 1 (extension into parametrium only) | 04 (Bladder infiltration)+ 1 (extension into parametrium only) |
Interpretations of observer 2.
| FIGO staging | Number of patients |
|---|
| Clinical examination | MRI (non contrast) | MRI – post contrast scan |
|---|
| 1b | 10 | 8 (no extension into parametrium) + 2 (extension into parametrium) | 7 (no extension into parametrium) + 3 (extension into parametrium) |
| 2a | 07 | 5 (extension into upper 2/3 vagina) + 2 (extension into parametrium) | 4 (extension into upper 2/3 vagina) + 3 (extension into parametrium) |
| 2b | 17 | 15 (extension into parametrium only) + 2 (bladder infiltration) | 15 (extension into parametrium only) + 2 (bladder infiltration) |
| 3a | 02 | 02 (extension into lower third vagina) | 02 (extension into lower third vagina) |
| 3b | 04 | 04 (extension into pelvic wall) | 04 (extension into pelvic wall) |
| 4 | 5 | 04 (bladder infiltration) + 1 (extension into parametrium only) | 04 (bladder infiltration)+ 1 (extension into parametrium only) |
Interruption of hypointense cervical stroma with or without hyperintensity in adjacent parametrium [Table/Fig-6,7], and interruption of the hypointense inner walls of urinary bladder [Table/Fig-8] and rectum along with loss of fat planes between the organs and the mass could be more easily and reliably identified than the extension of enhancement outside the confines of the cervix.
T2 weighted fat suppressed axial: a) and T1 fat suppressed post contrast axial image; b) showing cervical mass infiltrating into left parametrium. Also, note the T2 hypointense cervical stroma (white arrow) which is focally interrupted at the site of parametrial infiltration (black arrow in image a). Black arrow in image b depicts the speculated/pointed extension of the enhancement into the parametrium.
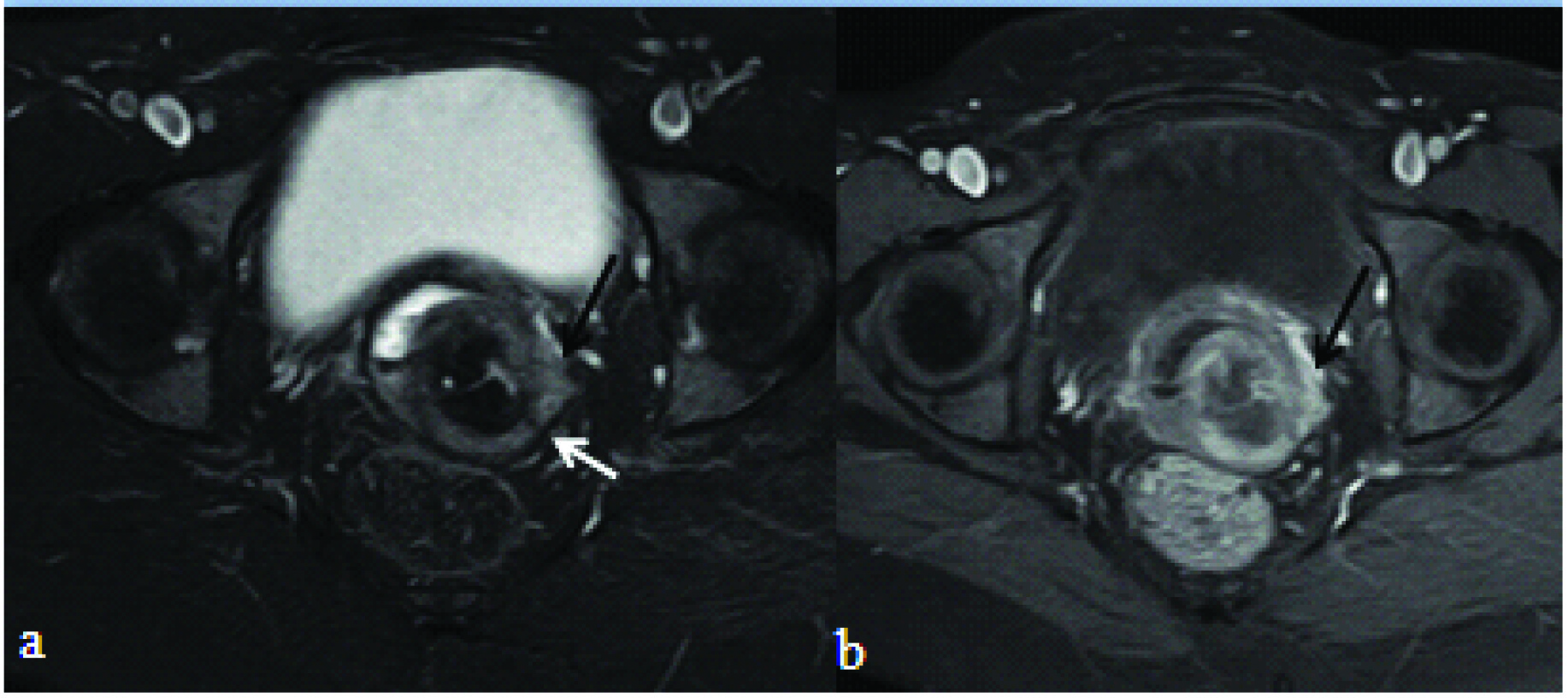
T2 weighted axial: a) and T1 fat suppressed post contrast axial image; b) showing cervical mass infiltrating into the left parametrium (white arrow).
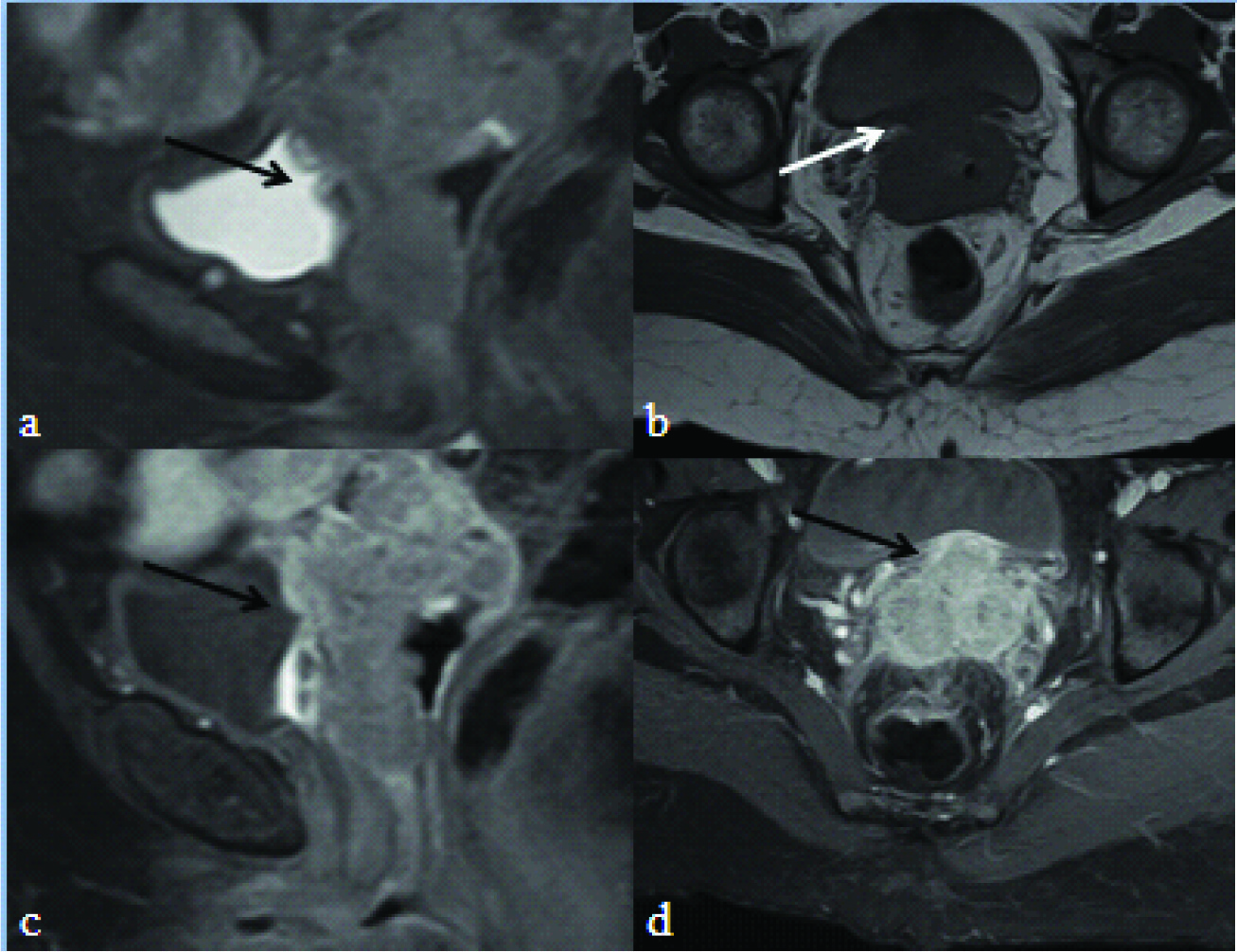
a) T2 weighted fat suppressed sagittal image showing cervical mass infiltrating into the urinary bladder (black arrow) with discontinuity of inner T2 hypointense bladder mucosa; b) T1 axial image showing loss of fat plane between the cervical mass and the urinary bladder and focal bladder wall thickening. Post contrast T1 weighted sagittal; c) and axial; d) fat suppressed image showing the enhancing cervical mass infiltrating into the bladder (black arrow).
Seventeen patients with a clinical staging of 1b and 2a underwent surgery and peroperatively two patients with stage 1b were confirmed to have parametrial extension. Of the seven patients with FIGO stage 2a, two cases also showed extension into parametrium. These findings were concordant with the non contrast MRI image interpretation of both the radiologists.
In all other patients who did not undergo surgery, both the noncontrast and post contrast MRI imaging findings were in agreement with each other including cases with extension into urinary bladder. No cases showed infiltration of rectum.
Of these four cases, which showed infiltration of the lateral pelvic wall [Table/Fig-9], three showed ipsilateral hydroureter secondary to ureteric infiltration.
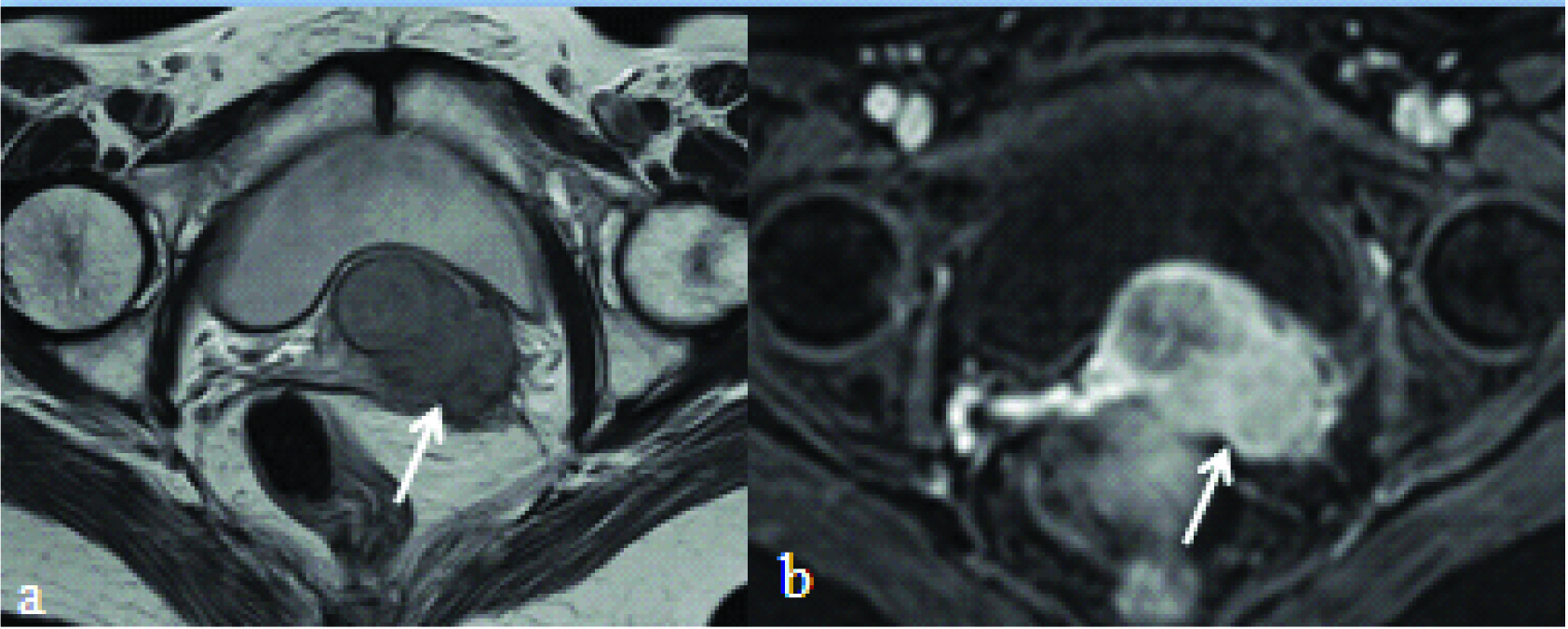
T2 weighted fat suppressed axial: a) and T1 fat suppressed post contrast axial image; b) showing cervical mass infiltrating into right lateral pelvis wall musculature (white arrow).
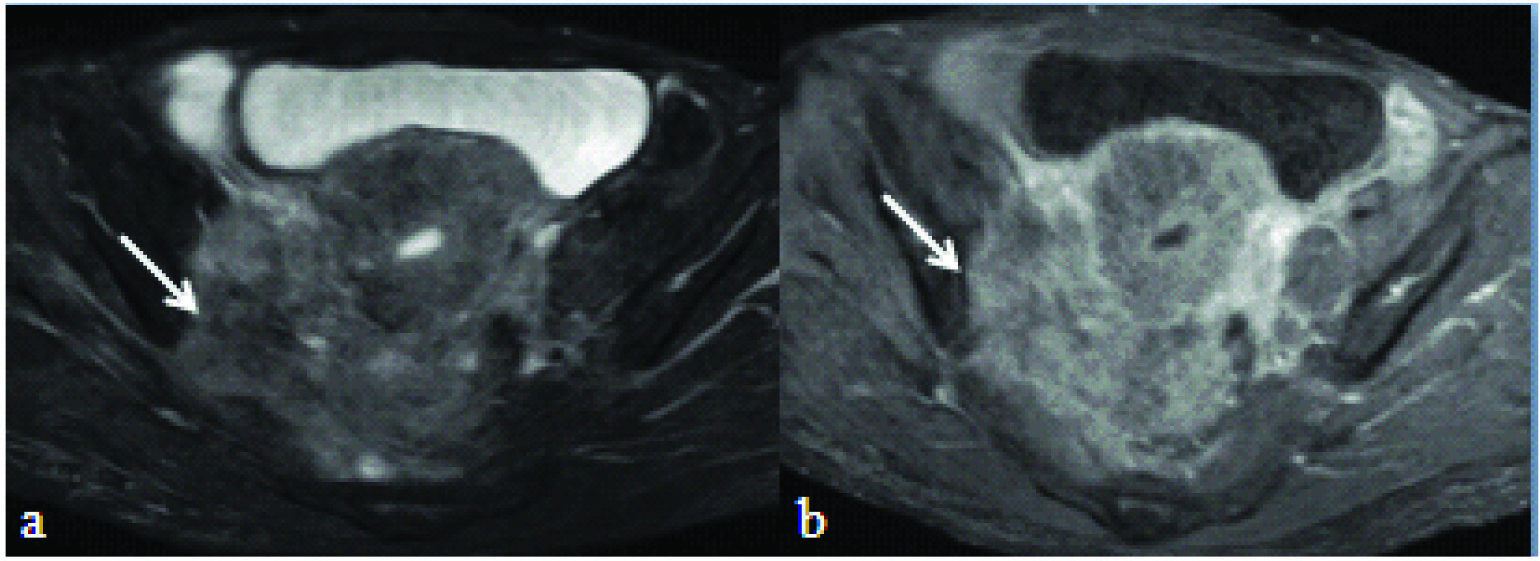
Of the 30 cases with parametrial and /or bladder infiltration, 15 cases showed bilateral parametrial infiltration which were also correctly identified on both non contrast and post contrast images. However, observers were of the opinion that it was easier and reliable to identify on non contrast T2 weighted images than post contrast images.
There was no difference between post contrast and non contrast images in pelvic lymph node assessment. Twenty out of 45 cases showed pelvic nodes (external, internal iliac and obturator nodes), most being subcentimetric. Nine of these patients showed enlarged lymph nodes and these were patients with carcinoma in advanced stages (urinary bladder/lateral pelvic wall/lower third vaginal involvement).
Discussion
Post contrast Spin Echo T1 weighted fat suppressed MRI imaging of pelvis is commonly performed in carcinoma cervix assessment.
However, in this study, it offered no distinctive advantage over and above the non contrast sequences, in either interpreting parametrial extension or bladder wall infiltration and most of the findings could be identified on non contrast images. This is in concurrence with studies by Hawighorst H et al., and Schiedler J et al., who found no value addition provided by this sequence [6,7].
Moreover, in comparison with non contrast images, post contrast images showed discrepancies in at least four cases which were interpreted as parametrial infiltrations, while non contrast and peroperative findings did not reveal such extension [Table/Fig-10]. This over staging effect of post contrast imaging is also described by Sala E et al., and Engin G et al., [11,12], however, Sala E et al., mentioned that similar effects may also be seen in T2 weighted images and dynamic post contrast scans.
T2 weighted axial: a) and T2 fat suppressed axial images; b) showing an endocervical mass (asterisk) with continuous ring of hypointense cervical stroma (white arrows) without parametrial infiltration; c) T1 post contrast fat suppressed axial image of the same patient showing bilateral parametrial enhancement (white arrows). This patient underwent surgery and peroperatively no parametrial extension was seen.
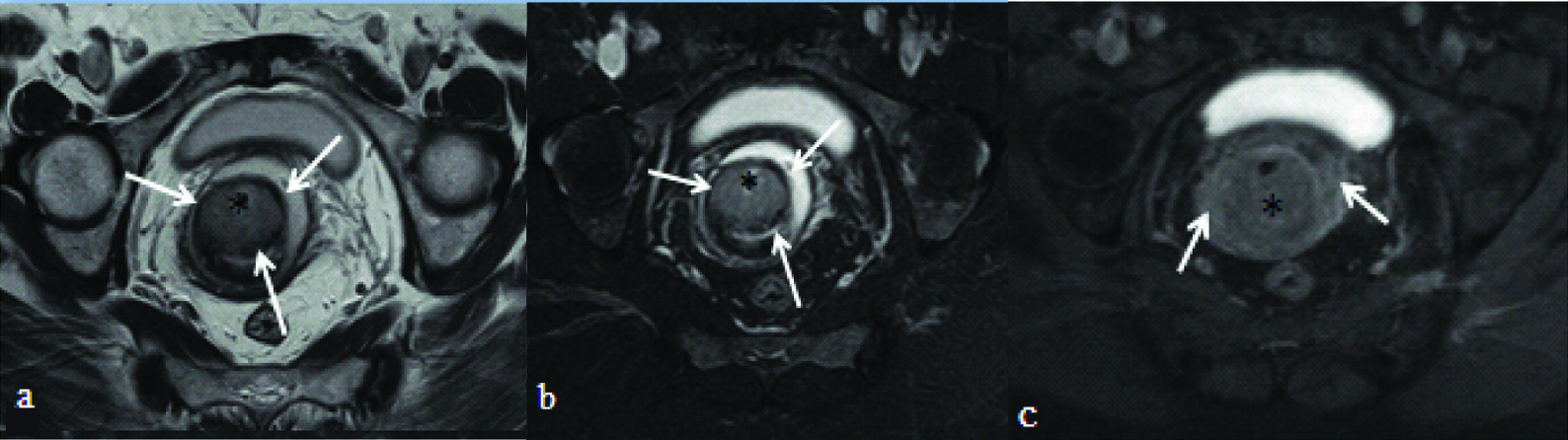
In the present study, it was reliable and easier to identify the loss of T2 hypointense cervical stroma than to delineate ill defined adnexal enhancement. Moreover, mild form of parametrial enhancement may also be seen due to inflammation. In addition, it is essential to delineate normally enhancing paracervical and uterine plexus of vessels, which often can be misinterpreted as infiltration into parametrium.
In the present study, both the observers were of the opinion that T2 weighted axial images were most suited to assess parametrial/ lateral pelvic wall extension. This is in agreement with studies by Freeman SJ et al., [13]. Preservation of inter-organ fat planes could be best assessed in T1 weighted images without fat suppression. Extension into urinary bladder was best assessed in T2 weighted sagittal images. However axial T2 weighted images with fat saturation were also beneficial. Utility of T1 weighted fat saturated images were limited to comparison with post contrast fat saturated images in identifying enhancement. Imaging in coronal plane offered no significant benefits.
Dynamic contrast enhanced imaging using T1 weighted 3 dimensional gradient protocols, though not used in this study, has been useful as a problem solving tool, whenever the findings on non contrast images are unequivocal. One such situation is to differentiate parametrial inflammation versus tumour extension. Another condition is when there is loss of fat plane with adjacent organs with doubtful infiltration on non contrast scans. Any small foci of infiltration into adjacent organs can be readily identified. In addition, dynamic post contrast studies are valuable in preinvasive disease when no lesion can be identified on T2 weighted images [14-16].
Based on the findings of this study, a compact MRI protocol for evaluation of carcinoma cervix, consisting of TSE T2 (Turbo Spin Echo- T2) in sagittal and axial (axial to plane of uterus) planes, TSE T2 with fat suppression and Spin Echo T1 axial imaging is recommended. Post contrast Spin Echo T1 weighted imaging provides no additional information. In case of ambiguity, dynamic post contrast imaging using gradient T1 weighted 3 dimensional imaging is recommended in selective cases. Adequate distension of vagina with aqueous jelly prior to MRI will allow for better delineation of the mass.
With this recommended protocol, there will be a reduction in the effective time of MRI acquisition and reduced usage of intravenous contrast. In a developing country like India, where carcinoma cervix is one of leading causes of mortality and morbidity, this means affordability for better management for poor and significant reduction in financial burden on the society.
Limitation
Peroperative findings were not available in all the cases in the present study. This is because the surgery as a primary modality of treatment is limited to cases with FIGO staging upto 2a stage. Many patients with parametrial invasion, infiltration into adjacent organs did not underwent surgery and hence could not be compared. The value of dynamic post contrast imaging was not assessed in this study.
Conclusion
Routine post contrast T1 weighted imaging provides no added advantage in comparison with non contrast T1 and T2 weighted imaging in evaluation of carcinoma cervix. Hence, it is suggested that routine Spin Echo post contrast imaging need not be part of the protocol in all such cases. However, only in selective cases with ambiguity on non contrast images, dynamic post contrast imaging may be used as a problem solving tool.
*FS – Fat Suppressed.
*FIGO – International Federation of Gynaecology and Obstetrics.