Leptospirosis, though worldwide in distribution, is found to be endemic in South East Asia [1]. The disease can present in any of the following epidemiological ways. The disease may present as a sporadic case in temperate regions [3,4]. It can even lead to vast spread and cause epidemics of leptospirosis [5,6]. In India, leptospirosis is known to be endemic in many states including the Southern states of India such as Karnataka [7]. The predominance of agriculture is the mainstay of economy in India, the presence of the subtropical climate that India enjoys, seasonal rainfall that may sometimes lead to flooding in some areas of the country are factors that make conditions favourable for leptospirosis to be endemic in India and also support its transmission.
Leptospirosis falls in the spectrum of diseases that present with acute febrile illness [1]. The clinical presentation may vary from mild forms like simple febrile illness to severe disease such as multiple organ failure [8]. In India, acute febrile illness is also the presentation of arthropod borne infectious diseases such as malaria, dengue, and chikungunya [6]. In such a scenario, leptospirosis presents a significant diagnostic challenge to the clinicians. The presence of other common endemic vector borne acute febrile illnesses along with a delay in the laboratory confirmation of the illness by serological tests makes the diagnosis and treatment even more problematic. Frequently, the treatment has to be initiated on the basis of clinical suspicion alone while the diagnostic tests are awaited.
In such a case, where the presentation is similar amongst many diseases with grossly different lines of treatment, and when there is a delay in serological diagnosis, other common tests may need to be evaluated to help suggest the diagnosis. Haematological investigations are routinely performed and the pattern of change of the haemograms frequently helps the clinician in differentiating the disease amongst the vast differential diagnosis of the febrile tropical diseases. While many studies have looked at biochemical biomarkers for several of these severe diseases [9], few studies are done on effects of leptospirosis on these haematological parameters [10,11]. The changes in the serial tests of complete blood counts have not been studied in the vast Indian population.
We, therefore, conducted this study to observe the serial changes in complete blood counts of leptospirosis patients. This may help detect factors that may be correlated to the severity of the disease. Determination of such factors and predictors of severity may not only suggest the diagnosis in the absence of the serological diagnosis but may also help guiding the management of the patient by ensuring timely intervention.
Materials and Methods
The retrospectively collected data was prospectively studied, which was of all the patients of leptospirosis admitted to medical wards of KMC Hospitals, at Attavar and Jyothi circle, Mangalore. All the cases of leptospirosis (confirmed by IgM ELISA) from January 2013 to September 2016 were enrolled in our study group. After obtaining institutional Ethical Clearance, demographic, clinical, serological and haematological findings (daywise, wherever done) were recorded till the discharge, transfer or death of the patients. The data was obtained from the medical records for the retrospective cases. The disease was categorised as severe if the following criteria was met: urine output less than 400 ml/day, serum creatinine greater than 1.5 mg/dl, serum urea greater than 71.4 mg/dl, total bilirubin greater than 3 mg/dl, hospital stay more than 10 days, stay in ICU or death. The cases were thus categorized as mild or severe disease. We studied the changes in complete blood counts from admission upto the discharge of the patient.
Statistical Analysis
Comparison between disease severity and haematological parameters were done using SPSS software version 13 (SPSS Inc. Chicago). The collected data was analysed using frequency, percentage, mean, median, standard deviation and Mann-Whitney test. A p-value less than 0.05 were considered significant.
Results
During the study period, 130 cases of serologically confirmed leptospirosis were included in the final analysis. Of these 92 (70.8%) were males and 38 (29.2%) were females. Mean age of the patients affected by the disease was 45±15 years. Majority of the patients were affected in the economically productive age group of 31-60 years. (31-40=22.3%, 41-50=21.5%, 51-60=23.8%) [Table/Fig-1].
Age distribution of leptospirosis cases in the study.
| Age in years | Frequency | Percentage |
|---|
| 20 and below | 9 | 6.9 |
| 21-30 | 11 | 8.5 |
| 31-40 | 29 | 22.3 |
| 41-50 | 28 | 21.5 |
| 51-60 | 31 | 23.8 |
| Above 60 | 22 | 16.9 |
| Total | 130 | 100.0 |
A total of 43 (33%) patients presented with mild disease while 87 (67%) patients had severe disease. All of the patients recovered in the study. Fever was the commonest (n=116) symptom followed by headache (n=54) [Table/Fig-2].
Showing the clinical presentation of leptospirosis in the study.
| Symptoms | No. of patients | Mean (Duration of symptoms) | Std. deviation |
|---|
| Fever | 116 | 4.59 | 2.696 |
| Headache | 54 | 4.22 | 2.470 |
| Myalgia | 38 | 5.08 | 2.705 |
| Vomiting | 31 | 3.03 | 2.689 |
| Pain Abd | 12 | 4.17 | 5.271 |
| Jaundice | 18 | 3.94 | 4.684 |
| Decreased Urine Output | 24 | 2.58 | 1.283 |
| Breathlessness | 18 | 3.33 | 1.572 |
| Loose Stools | 34 | 3.91 | 2.822 |
All the patients were followed till discharge and there were no death or transfer of any patient in this study.
Mean haemoglobin concentration showed a progressive decline in haemoglobin concentration (<12.5 gm/dl) from day 1 to day 7 in both mild and severe disease. The difference in the decline between mild and severe forms of the disease was not significant [Table/Fig-3].
Showing daywise variations in haemoglobin concentration (gm/dL) in mild and severe disease.
| Day | Haemoglobin inmild disease(gm/dl) | Haemoglobin insevere disease(gm/dl) | p-value |
|---|
| D1 | 12.2 | 12.7 | 0.668 |
| D2 | 11.85 | 11.45 | 0.425 |
| D3 | 11.1 | 11.1 | 1.000 |
| D4 | 10.8 | 10.4 | 0.510 |
| D5 | 10.95 | 10 | 0.294 |
| D6 | 10.2 | 10.4 | 0.970 |
| D7 | 9.3 | 10.25 | 0.219 |
| D8 | | 9.6 | |
| D9 | | 9.35 | |
| D10 | | 9 | |
| D11 | | 9.6 | |
Platelet counts were significantly low (p=0.002), (<1,50,000 cells/mm3) in severe disease between day 1 to day 3, dropping to 34,000 cells/mm3 on day 2 as compared to the counts in mild disease [Table/Fig-4,5]. The difference became insignificant from day 6 onwards.
Daywise variations in platelet count (/mm3) during the course of the Η Illness.
| Day | Platelet count inmild disease(/mm3) | Platelet count insevere disease(/mm3) | p-value |
|---|
| D1 | 137000 | 41000 | 0.000 |
| D2 | 113000 | 34000 | 0.003 |
| D3 | 94000 | 43000 | 0.002 |
| D4 | 93000 | 67000 | 0.537 |
| D5 | 110500 | 69000 | 0.053 |
| D6 | 115000 | 114000 | 0.617 |
| D7 | 113000 | 172500 | 0.215 |
| D8 | 220000 | 169000 | 0.843 |
| D9 | | 150000 | |
| D10 | | 124000 | |
| D11 | | 490000 | |
| Mean | 124437.5 | 133954.5 | |
Comparison of platelet counts (/mm3) in mild and severe disease over the course of illness.
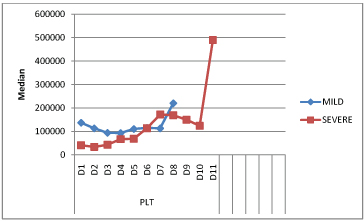
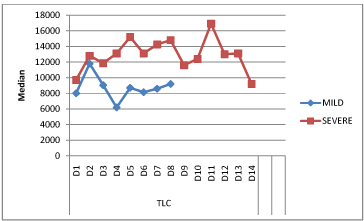
The mean total leucocyte counts showed a rise till day 2 in mild illness and upto day 5 in severe illness. Total leukocyte counts were significantly higher (p=0.0001), (> 11,000 cells/mm3) in patients with severe disease from day 4 to day 5 of the illness. A rise of total leucocyte count was noted again on D11 in severe disease followed by reducing trend thereafter [Table/Fig-6].
Comparison of total leukocyte counts (cells/mm3) in mild and severe disease over the course of illness.
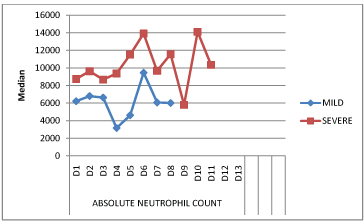
The mean absolute neutrophil count was at its lowest on day 4 in mild disease and on day 9 in severe disease. The counts were highest on day 6 in mild disease and on day 13 in severe disease [Table/Fig-7].
Comparison of absolute neutrophil counts (cells/mm3) in mild and severe disease over the course of illness.
Discussion
Leptospirosis manifests with a wide range of clinical features that overlap with those due to dengue and malaria, the infections that are common in this part of the country [1]. The disease is associated with haematological and biochemical abnormalities that complicate the disease process [9-11]. The disease manifests in mild and severe forms. We aimed at studying the variations in the haematological parameters particularly the complete blood counts associated with mild and severe forms of leptospirosis during course of the illness.
In our study, the mean age of the patients was 45±15.092 years. This was comparable to Esen S et al., who recorded a mean age of 47.3±15.7 years [12]. Similarly, as per Rajapakse S et al., the mean age of the sample was 44.49±14.42 years [13]. In a study by Karunanayake L et al., mean age was 42±15years [1].
In our study, majority of the patients were affected in the age group of 31-60 years. As per the study done by Karunanayake L et al., clustering was in the age interval of 30-59 years [1]. In the study by, Rajapakse S et al., clustering of cases was seen between 30–50 years of age [13].
In the study, 70.8% patients were males and 29.2% were females. In the study done by Esen S et al., 80% of the patients were males while 20% were females [12]. The study by Rajapakse S et al., had 86.6% males and 13.4% females [13]. The males and females affected in study by Karunanayake L et al., was 83.3% and 16.7% respectively [1]. The rural female workforce in India is 61.3% and 36.4% in Sri Lanka as per 2011 and 2013 Census respectively [14,15]. Therefore, having a larger population in India; a larger female population comes in contact with animals than those in Sri Lanka. Thus, Indian females may be at higher risk of developing leptospirosis which is reflected in our study.
In our study, the commonest symptom was fever (89.2%), followed by headache (41.5%), and myalgia (29.2%), vomiting (23.8%), breathlessness (13.8%), and jaundice (13.8%) were also found in our patients. By the studies performed by Esen S et al., and Rajapakse S et al., fever was present in 61.1% and 95.2% of patients respectively [12,13]. Headache was seen in 81.4% patients as per Rajapakse S et al., [13]. This symptom was not reported by Esen S et al., [12]. As per Esen S et al., and Rajapakse S et al., myalgia was seen in 65.7% and 88.3% respectively [12,13]. Vomiting was seen in 65.3%, respiratory symptoms seen in 72.1% and icterus noted in 75% patients in the study by Esen S et al., [12]. These findings were not reported by Rajapakse S et al., [13]. Both Esen S et al., and Rajapakse S et al., were studies from Sri Lanka [12,13]. A combined analysis of both the studies shows that fever was seen in 78.1% patients and myalgia in 77% patients. The variation in clinical presentation may be attributed to different serovars of Leptospira affecting India and Sri Lanka. Serovars found in India are pyrogenes, pomona, australis, utumnalis, hebdomadis, hardjo, icterohaemorrhagiae, canicola, javanica [7]. Serovars found in Sri Lanka are Icterohaemorragiae, Ceylonica, Geyaweera, Weerasinghe, Ratnapura, Gem, Alice, Autumnalis, Canicola, Hebdomadis, Lanka, Grippotyphosa, Javanica, Pomona, Bangkinang, Bulgarcia, Ricardi, Pyrogenes [16].
In our study, the mean platelet counts were low (<1,50,000 cells/mm3) at presentation in both mild and severe forms of the disease [Table/Fig-5]. This correlated with the results of other studies [10,11-13,17]. According to a study, mean platelet count less than 46,000 cells/mm3 was associated with sepsis which is severe form of leptospirosis [11]. The present study encountered a markedly low mean platelet count (<50,000 cells/mm3) in severe disease at presentation. A further decline was noted till day 3 and day 4 in mild disease followed by gradual increasing trend and normalizing by day 8 of recovery [Table/Fig-4]. Similar trend of platelet count variation during disease course was observed in another study [6]. Severe thrombocytopenia is associated with renal failure and high mortality [10,11]. The proposed mechanisms suggested for thrombocytopenia include loss of integrity of the vasculature due to direct effect of the organism, bone marrow suppression and decreased production, direct activation of platelets, peripheral destruction or increased consumption [10,11-13,18].
In our study mean haemoglobin concentration showed a progressive decline in both, mild and severe disease. However, there was no significant difference in the decline between the mild and severe forms. De Silva NL et al., reported a decline in haemoglobin over the course of the disease [9]. Direct toxic effect of the leptospires may cause the drop in haemoglobin [10].
The mean total leucocyte counts showed a rise till day 2 in mild illness and upto day 5 in severe illness. Total leukocyte counts were significantly (p=0.0001) higher in patients with severe disease from day 4 to day 5 of the illness than that in mild disease. This was also observed by Craig SB et al., [10]. De Silva NL et al., in their study also noted a similar fall in the total leucocyte count for the first five days followed by a gradual rise [9]. A rise of total leucocyte count was noted again on D11 in severe disease followed by reducing trend thereafter. However, no similar findings were seen in any of the studies reviewed by us. None of the patients had leukopenia, a feature commonly associated with dengue [19]. We agree with De Silva NL et al., that in a setting of febrile illness with thrombocytopenia, normal to high leukocyte count helps in differentiating leptospirosis from dengue [9]. Majority of severe cases had neutrophilia as compared to milder form. This mechanism may facilitate phagocytosis and killing of the pathogen [10]. Lymphopenia was associated with leptospirosis [20,21]. Lymphopenia was observed in both mild and severe disease cases in our study.
Limitation
Smaller sample size may explain the statistically insignificant neutrophilia and lymphopenia. Studies involving larger sample size may throw a light onto this aspect.
Conclusion
Complete blood counts are among the routinely carried out investigations in patients with leptospirosis. Haemoglobin level showed a progressive decline during the disease course which may help to differentiate from dengue. Thrombocytopenia, especially if marked, is associated with disease severity thus may help the treating physician to categorise the disease severity for better management. Thrombocytopenia in a setting of normal to high total leukocyte count should prompt towards a differential diagnosis of leptospirosis.