Vitiligo is the most common depigmentation disorder where the functioning melanocytes are selectively destroyed resulting in depigmentation of the skin, hair and mucosal surfaces [1]. Vitiligo affects about 0.5% to 1% of the population, with equal incidence in men and women. Average age of onset is estimated to be 24 years [2,3] and there is no difference in the rate of occurrence according to skin type or race [4]. Several aetiological factors have been proposed [5,6] for which the most convincing evidence involves a combination of unknown environmental [7], genetic and immunological factors interacting to contribute in autoimmune melanocyte destruction [1].
The most prominent feature in vitiligo is modification of melanocytes at the dermoepidermal junction. Sections stained with H&E stain are often very subtle and may be unconvincing. Special staining procedures like the DOPA reaction may reveal viable and functional melanocytes. Fontana-Masson staining may also help in attainment of a definite diagnosis. It is observed that depigmentation in vitiligo is associated with inflammatory infiltrates of T cells and macrophages [8]. In progressive disease, the CD4+:CD8+ ratio is decreased among skin-infiltrating T cells and CD8+ T cells isolated from vitiligineous skin are cytotoxic to melanocytes [9]. The application of multicolor flow cytometry technologies discloses multiple phenotypically distinct subsets of T-cells in the peripheral blood of human. The flow cytometrically determined CD8+ T-cell counts were found to be significantly higher and CD4+:CD8+ ratio decreased significantly in Generalized Vitiligo (GV) patients as compared with controls, in few studies [10,11]. The present study was conducted with the aim to study the role of cellular immunity in the pathogenesis of vitiligo, by estimation of CD8+ and CD4+ T lymphocyte counts by flow cytometric analysis, in histopathologically and histochemically proven cases of vitiligo.
Materials and Methods
The present prospective study was done in two years time duration, which included evaluating skin biopsies of both male and female vitiligo patients of all age groups, received in the Pathology Department from patients coming in the Skin Department of our institute which is a tertiary care centre of Amritsar, Punjab, India. The study included 40 patients of proven cases of vitiligo diagnosed on H&E stained sections and DOPA reaction. Patients with autoimmune diseases were excluded from this study. Informed consent was taken from all patients prior to enrolment in the study. The procedures followed were in accordance with the Ethical Standards of the Institutional Research Committee.
The blood samples from 40 proven cases of vitiligo along with 10 random control subjects were collected in EDTA vials for the estimation of CD4+ and CD8+ counts. BD ACCURI C6 four colour flow cytometer using fluorochromes- PER-CP, APC, PE and FITC was used. Antibodies used were CD45, CD3, CD8 and CD4. On Flow Cytometer, firstly dot plot diagram was plotted using CD45 on X axis and Side Scatter (SSC) on Y axis. From this graph, lymphocyte population was gated and this population was plotted with CD3 on X axis and SSC on Y axis. CD3+ population was then further gated and plotted on another graph with CD8+ on Y axis and CD4+ on X axis. Then CD4+ and CD8+ were counted and CD4+: CD8+ ratio was estimated.
Statistical Analysis
Continuous data were summarized as Mean±SD (standard deviation) while discrete (categorical) in number and percentage (%). Continuous groups were compared by Student’s t-test. A two-tailed p<0.05 was considered statistically significant.
Results
The age of patients included in the present study varied from 8 years to 60 years. The maximum numbers of patients were between 11-20 years of age. The least number of patients were in age group of 0-10 years. Out of total 40 cases, 55% patients were males and 45% were females. A 60% of patients were from urban area while 40% of patients were from rural area. The most common site of biopsy in patients was lower limb constituting 47.5% of cases followed by face and upper limb constituting 20% and 17.5% cases respectively. Other sites included were neck, chest and abdomen.
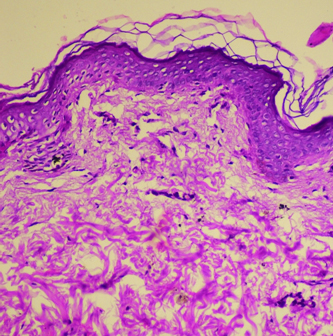
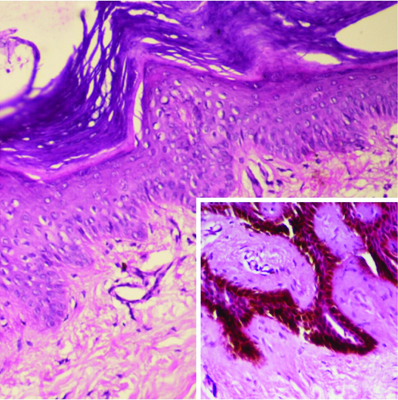
The duration of vitiligo in 37.5% of patients was between zero to six months. This was followed by 6-12 months of duration in 20% cases and more than five years duration in 20% cases. The disease was found to be progressive in 32 (80%) patients while it was stable in 8 (20%) of patients. All these 40 cases revealed loss of melanin pigment and melanocytes on H&E and DOPA reaction [Table/Fig-1, 2]. Perivascular lymphocytic infiltrate consisting mainly of lymphocytes was found in 65% of cases.
Microphotograph showing absence of melanin pigment as well as melanocytes in epidermis in a vitiligo case with patch on lower limb (H&E, 20X).
Microphotograph showing absence of melanin as well as melanocytes in epidermis in a vitiligo case with patch on foot (DOPA, 20X).
Inset shows granular melanocytes in the control tissue (DOPA, 40X).
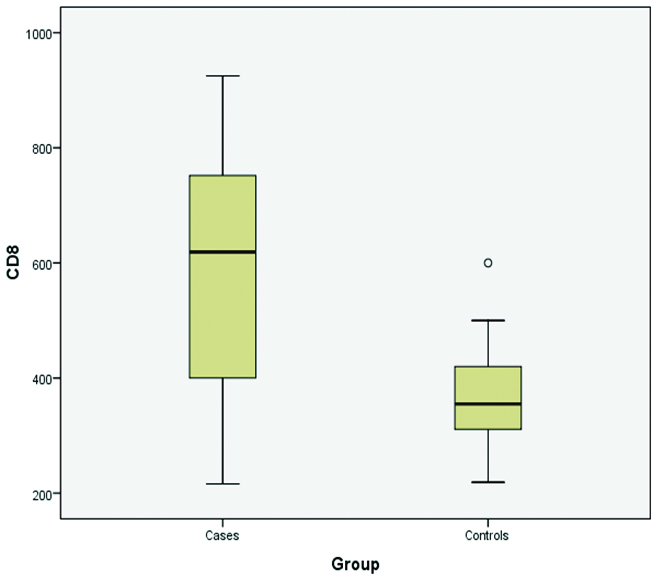
CD8+ counts
Maximum number of patients of vitiligo (35%) had CD8+ counts within range of 600-800×106. This was followed by 11 (27.5%) cases in which CD8+ counts were between the range of 200-400×106, which is the lower normal range. The lowest CD8+ count recorded in vitiligo patients was 216 while highest CD8+ count recorded was 925. Six out of 10 controls (60%) had CD8+ counts in the range of 200-400. Minimum CD8+ count recorded in control groups was 219 while maximum CD8+ count was 600 [Table/Fig-3].
Distribution of CD8+ counts in vitiligo patients and controls.
Distribution of CD4+ counts in vitiligo patients and controls.
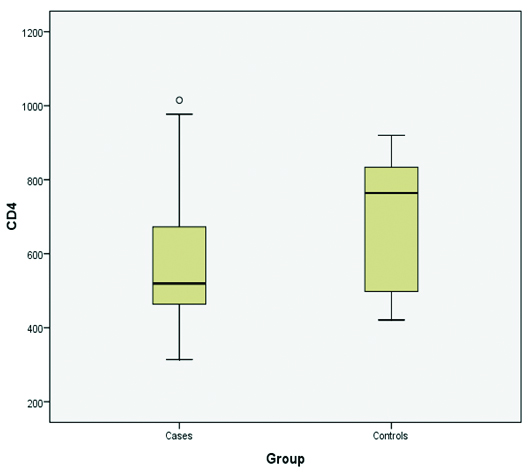
CD4+ counts
Maximum number of patients (60%) had CD4+ counts within the range of 400-600×106 which is the lower normal range. Nearly, 22.5% of patients had CD4+ counts in range of 600-800×106. Three patients of vitiligo had reduced CD4+ counts between the range of 200-400×106. The highest CD4+ count was 1015 while the lowest CD4+ count recorded was 314. Four out of 10 controls (40%) had CD4+ counts between the range of 800-1000 ×106. Maximum CD4+ count recorded was 920 while minimum CD4+ count recorded was 421 [Table/Fig-4].
CD4+:CD8+ ratio
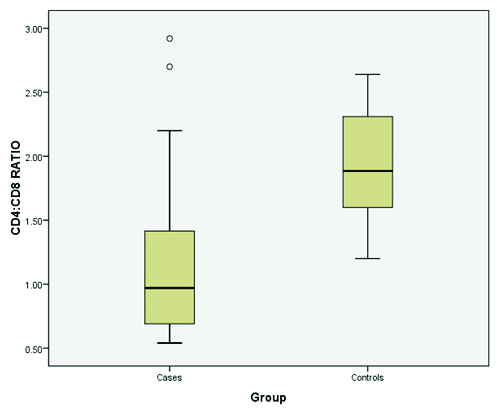
CD4+:CD8+ ratio was altered in vitiligo patients. This ratio was found to be decreased to below than one. Lowest CD4+:CD8+ ratio was found to be 0.54 while highest ratio was recorded as 2.92 [Table/Fig-56,7 and 8]. The lowest CD4+:CD8+ ratio in control subjects was found to be 1.2 while the highest was recorded to be 2.64 [Table/Fig-9,10]. Maximum decrease in CD4+: CD8+ ratio was observed in 50% cases having age of onset within 1-20 years of age. This was followed by patients in range of 21-40 years. Only one patient aged 43 years had decreased CD4+ counts with increased CD8+ counts.
Comparison of CD4+:CD8+ ratio in vitiligo patients and controls.
| CD4+:CD8+ ratio | Vitiligo patients | p-value | Control subjects |
|---|
| No of patients | Percentage (%) | | No of controls | Percentage (%) |
|---|
| <1 | 22 | 57.5 | < 0.05 | 0 | 0 |
| 1-2 | 14 | 35 | 6 | 60 |
| 2-3 | 4 | 7.5 | 4 | 40 |
| >3 | 0 | 0 | 0 | 0 |
| Total | 40 | 100 | 10 | 100 |
Distribution of CD4+:CD8+ ratio in vitiligo patients and controls.
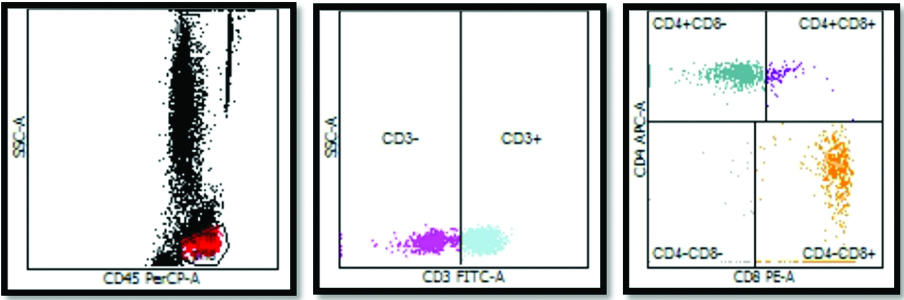
Flow cytometric analysis of vitiligo patient with decreased CD4+:CD8+ ratio.
a) Dot plot diagram with CD45PERCP-A on X- axis and SSC-A on Y axis. The lymphocyte population is shown in red dots. This population was then gated and plotted on second graph.
b) CD3 FITC-A is plotted on X-axis with SSC-A on Y axis. CD3+ population is then gated.
c) Using CD8 PE-A on X-axis and CD4+ APC-A on Y axis, CD4+ and CD8+ cells are counted in this patient CD8+ counts are greater as compared to CD4+ counts leading to decreased CD4+:CD8+ ratio.
Absolute values and percentages of each T cell count and ratio in vitiligo patients as calculated by flow cytometry.
| Parameter | Percent | Value/Absolute Count |
|---|
| Lymph Events | | 2499 |
| CD3+ | 80.23 | 1270.36 |
| CD3+CD8+ | 51.38 | 813.54 |
| CD3+CD4+ | 30.25 | 479 |
| CD3+CD4+CD8+ | 2.92 | 46.25 |
| CD45+ | | 1583.36 |
| CD4+:CD8+ ratio | | 0.59 |
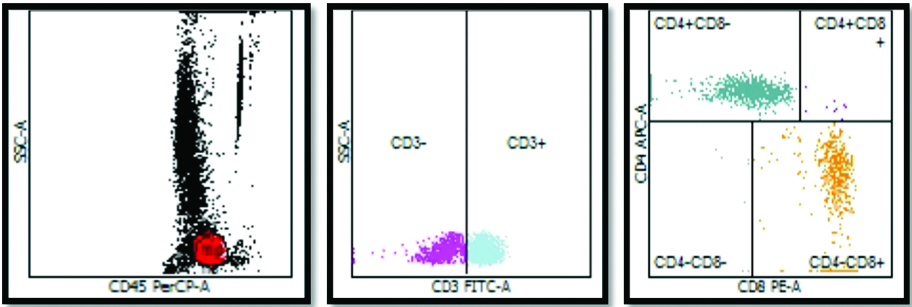
Flow cytometric analysis of control subject with normal CD4+:CD8+ ratio.
a) Dot plot diagram with CD45PerCP-A on X- Axis and SSC-A on Y Axis. The lymphocyte population is shown in red dots. This population was then gated and plotted on second graph.
b) CD3 FITC-A is plotted on X-Axis with SSC-A on Y Axis. CD3+ population is then gated.
c) Using CD8+ PE-A on X-axis and CD4+ APC-A on Y axis, CD4+ and CD8+ cells are counted. In this control subject CD4+ counts are greater as compared to CD4+ counts leading to normal CD4+:CD8+ ratio.
Absolute values and percentages of each T cell count and ratio in control subjects as calculated by flow cytometry.
| Parameter | Percent | Value/Absolute count |
|---|
| Lymph Events | | 2581 |
| CD3+ | 70.86 | 1171.01 |
| CD3+CD8+ | 26.81 | 443.05 |
| CD3+CD4+ | 44.13 | 729.24 |
| CD3+CD4+CD8+ | 0.35 | 5.76 |
| CD45+ | | 1652.48 |
| CD4+:CD8+ ratio | | 1.65 |
QC Messages
Manual Gate is in effect.
% T-Sum is: 0.08
4/8 ratio is: 1.65
Maximum decrease in CD4+: CD8+ ratio was observed in 50% cases with disease duration between zero to six months. Minimum duration of vitiligo reported was 20 days. Maximum disease duration reported was 30 years in which CD4+:CD8+ ratio was decreased. Most of the patients with normal CD4+:CD8+ ratio had disease duration of more than five years. A 22.23% with normal CD4+:CD8+ ratio had disease duration zero to six months while 3 (16.67%) patients had disease duration of 6-12 months.
Discussion
In vitiligo patients, there are several theories to suggest the aetiopathogenesis of the disease. Role of cellular immunity is one of the suggested theories [1]. Hence, the estimation of CD4+ and CD8+ T cells might be useful to support this hypothesis.
In the present study, majority of patients of vitiligo were in the age group of 11-20 years and least were in age group of >50 years. The age distribution in case study conducted by Shah H et al., had similar findings with majority of the patients between 11-20 years age group [12].
Males were affected more than females with male: female ratio 1.2:1. This was consistent with study done in 2008 by Handa S and Kaur I with male:female ratio 1.19:1 [13]. Equal sex incidence was found in studies conducted by Kar PK and Dogra S et al., [14,15].
Vitiligo constituted 16 cases from rural and 24 number of cases from urban areas in our study. However, in a study conducted by Shah H et al., in rural and urban areas of Bhavnagar, Gujarat, India showed 78% vitiligo cases in urban areas and 22% in rural areas [12].
Most common site of biopsy for vitiligo cases in our study was lower limb. This was similar to studies conducted by Kar PK and Martis J et al., where most common site of biopsy was also lower limb [14,16].
In this study, maximum number of patients (37.5%) presented within six months of onset of the vitiligo lesion. These were followed by the patients who had disease duration of 6-12 months and constituted 8 (20%) cases. Patients having disease duration of more than five years were 8 (20%) in number. This was similar to a study done by Shankar DS et al., where the 40% of patients had disease duration less than six months while 16.25% of patients had disease duration between 6-12 months [17].
All the 40 clinically suspected cases of vitiligo on histopathology demonstrated loss of melanin pigment and melanocytes. Perivascular lymphocytic infiltrate consisting mainly of lymphocytes was found in 65% of cases. In early evolving lesion of vitiligo, Haematoxylin and Eosin stained (H&E) section show basal hypomelanosis along with mild lymphocytic inflammatory infiltrate in papillary dermis. An established lesion of vitiligo shows scant inflammatory infiltrate, complete loss of melanocytes along with complete loss of epidermal pigmentation.
Special stains i.e., DOPA reaction for the confirmation of absence of melanocytes was used which showed complete absence of melanin pigment and melanocytes in cases of vitiligo while in the control there was presence of melanin and melanocytes. Melanocytes had characteristic appearance as dark granules in the cytoplasm as well as covering the nucleus. This was consistent with the study done by Hu F et al., which stated that DOPA positive cells in normal skin appear to be solid black with granularity [18]. Cytoplasmic granularity is an important requisite for a true positive DOPA reaction. In the vitiligenous areas, there are no true black cells with granularity but some round, ovoid, stellate shaped cells may appear grey or black having no granules in the cytoplasm. These may be referred to as “inactive melanocytes” [18].
CD4+ and CD8+ counts estimated in vitiligo patients by flow cytometry, which showed decrease in CD4+ counts and increase in CD8+ counts. Mean values of CD3 counts were observed to be increased. These findings were found to be similar in previous studies done by Grimes PE et al., where all the 20 patients of vitiligo showed decreased CD4+ counts and increased CD8+ counts as compared to 16 controls [19].
A study by Halder RM et al., compared aberrations in T cells by flow cytometry in 25 patients of vitiligo and 25 normal controls [20]. Results showed decreased Helper T cells and moderate increase in suppressor T cells in patients of vitiligo as compared with controls. In a study done in China, CD8+ cells were increased in 14.80% of patients of progressive vitiligo, 13.46% of patients with stable vitiligo and 8.66% of controls. CD4+ cells were decreased in progressive vitiligo patients. Study done in 2013 by Dwivedi M et al., also showed decrease in CD4+ counts with increase in CD8+ counts [10].
In the present study, CD8+ counts were increased in 55% of vitiligo patients. These counts were within upper normal range. In these patients, CD4+ counts were decreased to lower normal range thus, altering the CD4+: CD8+ ratio. This increase in CD8+ counts was found to be statistically significant as p-value was <0.001.
CD4+ counts were found to be significantly decreased in various studies. In present study, CD4+ counts was found to be significantly decreased in vitiligo patients when compared with healthy controls (p-value=0.031 which is less than 0.05). This finding is consistent with a study done by Sheneef A et al., who also observed significant decrease in CD4+ count with p-value = 0.01 [11]. The study done by Lili Y et al., and Hegab DS et al., also showed similar results [21,22].
CD4+:CD8+ ratio was found to be altered in 55% of cases of vitiligo. In these patients, the ratio was below one. In rest of the patients, the ratio was within the normal range. In none of the patients, the CD4+:CD8+ ratio was increased above three. CD4+:CD8+ ratio was found to be statistically significant decreased in vitiligo patients as compared with the control group with p-value<0.001 which is less than 0.05. These findings were consistent with the previous studies done by Grimes PE et al., in 1986 and Oggs et al., [19,23]. The CD4+: CD8+ ratio was altered in the vitiligo patients in their studies with increased CD8+ counts. A statistically significant decrease in CD4+: CD8+ ratio was also found in vitiligo patients as compared with controls with p-value <0.001 in a study done by Dwivedi M et al., [10]. However, a study done by Pichler R et al., showed increased CD4+:CD8+ ratio which might be due to association of autoimmune thyroid disease in 16 of the patients included in the study [24].
Maximum decrease in CD4+ counts with increase in CD8+ counts were observed within age group of 1-20 years followed by age group of 21-40 years. However, in the age group of 41-60 years, the CD4+: CD8+ ratio was observed to be normal. This finding is consistent with the study done by Dwivedi M et al., who observed significant decrease in CD4+ counts in age group 1-20 years as compared to 41-60 years but no significant difference was observed as compared with the age group 21-40 years [10].
Significant decrease in CD4+: CD8+ ratio was observed in patients who had recent onset of vitiligo lesion. Maximum number of patients who had decreased ratio had disease duration within six months. This was followed by the patients who had disease duration of six months to one year. Those patients who had disease duration of more than five years had normal CD4+:CD8+ ratio. Only two patients having disease duration of 10 years and 20 years had decreased CD4+: CD8+ ratio. This might be due to recent onset of new vitiligo lesions in previously stable vitiligo. But, this comparison was statistically not significant in this study. However, the study done by Grimes PE et al., observed statistically significant decrease in Helper T cells among patients with a disease duration of less than one year (p<0.001) [19].
Limitation
The present study is limited by less number of cases of vitiligo. Therefore, more number of studies with bigger sample size is required to achieve an improved understanding of immunopathogenesis of vitiligo.
Conclusion
Diagnosis of vitiligo is based on clinical findings as well as histopathology. Histochemical techniques i.e., DOPA are confirmatory. Alteration in CD4+ and CD8+ counts might play a role in the pathogenesis of vitiligo. Increased CD8+ counts have role in destruction of melanocytes. Hence, the estimation of CD4+: CD8+ ratio might be helpful in determining the immunopathogenesis of vitiligo. The estimation of this ratio might be helpful in cases of early vitiligo and in cases having vitiligo patch on face where patients are reluctant to give biopsy due to cosmetic reasons.