Cancer is one of the most serious and dreadful diseases prevalent today and is one of the leading causes of death worldwide. There were 14.1 million new cases and 8.2 million cancer related deaths in 2012, compared to 12.7 and 7.6 million respectively in 2008 [1]. Chemotherapy plays a pivotal role in the management of cancer. However, toxicities and side effects are the limiting factors of chemotherapy, resulting in poor quality of life in these patients, compelling them to drop the treatment schedules. Among the most common and serious side effects of chemotherapy, nausea and vomiting are ranked first and fifth respectively [2].
CINV is experienced by most patients in the first 24 hours (acute) while delayed CINV in 24-120 hours is experienced by 30%-60% of patients taking moderately or highly emetogenic chemotherapeutic agents [3]. Successful control of CINV could help to improve the quality of life and ability to perform daily activities and ensure completion of all schedules of cancer chemotherapy.
Therapeutic advances in development of antiemetic drugs have played key role in improvement of quality of life of patients undergoing cancer chemotherapy. Dopamine antagonists, corticosteroids, antihistamines, benzodiazepines and cannabinoids were the commonly used antiemetics. The concept of antiemetic prophylaxis was revolutionized by the introduction of serotonin receptor antagonists in 1990s which has now become one of the most important group of drugs for prevention of CINV [4]. First generation 5HT3 receptor antagonists, ondansetron, granisetron, dolasetron and tropisetron have shown comparable efficacy in preventing acute CINV, but they have limited effect on delayed CINV [4]. The second generation 5HT3 receptor antagonist, palonosetron, is a potent and selective antagonist with high affinity for 5HT3 receptors and has a long half-life [5].
Hence, the present study was conducted to compare the antiemetic effect and safety of palonosetron versus ondansetron in South Indian patients receiving first dose chemotherapy with moderately or highly emetogenic chemotherapeutic agents during acute phase (0-24 hours), delayed phase (24-120 hours) and overall period (0-120 hours).
Materials and Methods
In this prospective observational study, 212 patients diagnosed with malignancy and scheduled to receive first dose of moderately or highly emetogenic cancer chemotherapy, prescribed either ondansetron or palonosetron as antiemetic prophylaxis were enrolled in each treatment arm. Study duration was 12 months from January 2013 to January 2014. Sample size was calculated by substituting p-value at 80% power from the reference study done by Gralla R et al., [8]. Ethical clearance was obtained from the Human Ethics Committee of the institution.
Patients with GIT malignancies presenting with nausea or vomiting, pregnant and lactating females, patients who have known allergy or reported severe side effect to any of the study drugs and patients on radiation therapy were excluded.
Patients attending outpatient wing of radiotherapy department during the study period were screened before administration of their first chemotherapeutic schedule and those who satisfied the inclusion criteria were enrolled in the study. A written informed consent was obtained from willing patients. The antiemetic prophylaxis was prescribed by the treating clinical oncologist. Palonosetron was given in the dose of 0.25 mg IV whereas, ondansetron was administered 8 mg IV. Both drugs were given half an hour before chemotherapy. General examination was performed and results of laboratory investigations were collected from medical records as part of routine examination. Patients were provided with a daily diary/proforma to record the number of vomiting episodes, intensity and duration of nausea and to record the use of rescue medications at home for emesis control for five days following chemotherapy. After discharge from the hospital, patients were followed up telephonically to collect the data regarding nausea and vomiting and to ensure that the diary has been completed accurately and were asked to bring the diary on the next visit.
Data during first 24 hours following chemotherapy was used to assess acute CINV and 24 to 120 hours following chemotherapy to assess delayed CINV. Assessment of CINV was also done in overall period (0-120 hours). The data regarding number of episodes, severity of CINV and usage of rescue medication were collected from the patients diary and grade of nausea and vomiting were defined using NCI-CTCAE (VERSION 3.0) [10]. The outcome parameters were “complete response” (no emesis episode and no use of rescue medication or no more than Grade 1 nausea), “partial response” (≤1 episode emesis during the entire five day period (Grade 1 vomiting), no use of rescue medication or Grade 2 nausea) and “treatment failure” (need of rescue medication or Grade 2 vomiting or > Grade 2 nausea). Proportion of patients with nausea and vomiting during acute, delayed and overall period in both the study groups were compared.
Statistical Analysis
Data entry and analysis was done with the help of Excel 2013 and SPSS 16.0 statistical software (IBM). Performance status was assessed using NCI-CTCAE grading. Chi-square test was used to compare the difference in clinical response between the two groups. A p-value <0.05 was considered statistically significant.
Results
A. Demographic profile, age and gender distribution
The age range of patients included in the study was between 18 and 80 years. Most of the patients were above 50 years. Sixty eight (64.2%) patients in ondansetron group and 63 (59.4%) in palonosetron group were above 50 years [Table/Fig-1]. There was no significant difference in the age group between the two treatment groups (p-value= 0.480).
Frequency (f) and percentage (%) distribution of patients based on age in the two groups.
| Age group (years) | Drugs |
|---|
| Ondansetron (n=106) | Palonosetron (n=106) | Total (212) |
|---|
| (f) | (%) | (f) | (%) | (f) | (%) |
|---|
| <50 | 38 | 35.8 | 43 | 40.6 | 81 | 38.2 |
| >50 | 68 | 64.2 | 63 | 59.4 | 131 | 61.8 |
(Chi-square = 0.499, p-value = 0.480)
There were 46 males (43.4%) and 60 (56.6%) females in the ondansetron group and 55 males (51.9%) and 51 (48.1%) females in the palonosetron group [Table/Fig-2]. Comparison of gender distribution showed no significant difference between two groups (p-value = 0.216).
Frequency (f) and percentage (%) distribution of patients based on gender in the two groups.
| Gender | Drugs |
|---|
| Ondansetron(n=106) | Palonosetron(n=106) | Total (212) |
|---|
| (f) | (%) | (f) | (%) | (f) | (%) |
|---|
| Male | 46 | 43.4 | 55 | 51.9 | 101 | 47.6 |
| Female | 60 | 56.6 | 51 | 48.1 | 111 | 52.4 |
(Chi- square = 1.532, p-value = 0.216)
B. Clinical Response
Comparison of nausea in ondansetron and palonosetron group.
A total of 29 (27.4%) patients had acute nausea in ondansetron group whereas, in palonosetron group 24 (22.6%) patients had nausea during acute phase. Forty one (38.6%) patients had nausea during delayed period in ondansetron group whereas, 26 (24.5%) patients experienced nausea during delayed period in palonosetron group. A total of 47 (44.3%) patients had nausea during overall period in ondansetron group compared to 34 (32.1%) patients in palonosetron group [Table/Fig-3].
Percentage distribution of patients based on the presence of nausea during acute, delayed and overall phases in the two groups.
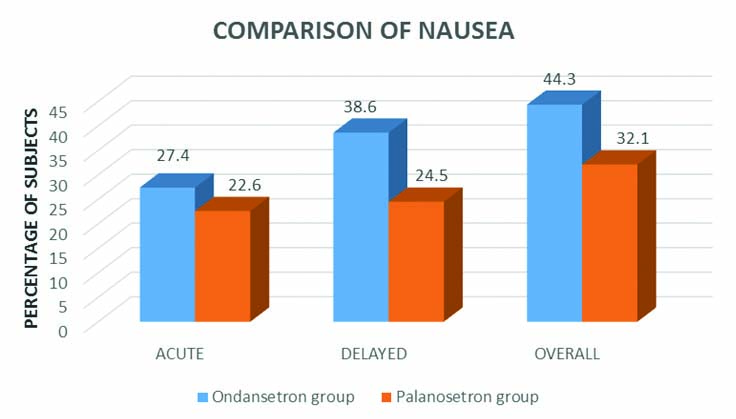
The difference in incidence of nausea among the two groups was statistically significant only during delayed phase (p = 0.027). However, there was no statistically significant difference in the incidence of nausea during acute phase (p=0.428) or overall phase (p=0.066) between the groups.
Comparison of vomiting in ondansetron and palonosetron group: Twenty one (19.8%) patients had acute vomiting in ondansetron group whereas, in palonosetron group 11 (10.4%) of the patients had acute vomiting. Thirty one (29.2%) patients experienced delayed vomiting during treatment in ondansetron group whereas, in palonosetron group 14 (13.2%) of the patients had delayed vomiting. A total of 37 (34.9%) patients had overall vomiting in ondansetron group whereas, in palonosetron group 19 (17.9%) patients had overall vomiting [Table/Fig-4].
Percentage distribution of patients based on the presence of vomiting during acute, delayed and overall phases in the two groups
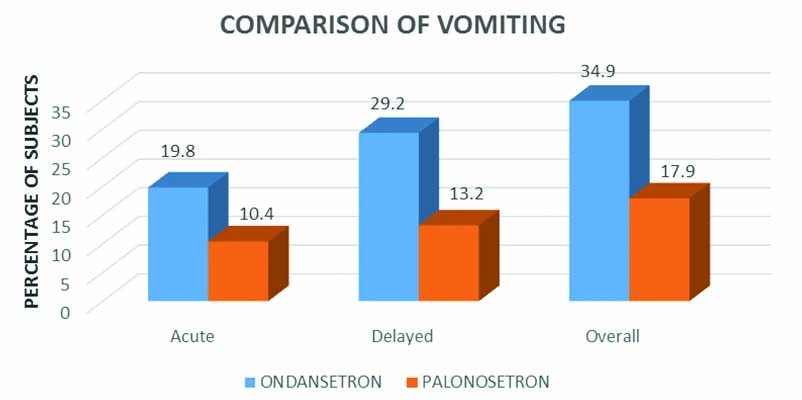
The difference in incidence of vomiting among the two groups was statistically significant during delayed phase (p-value=0.004) and overall period (p-value=0.005). However, there was no statistically significant difference in the incidence of acute vomiting (p-value=0.055) between the groups.
Comparison of response to antiemetic prophylaxis: Complete response during acute phase in ondansetron group was 80.2%, while for palonosetron it was 89.6%. During delayed phase ondansetron and palonosetron produced complete response in 70.8% and 86.8% patients respectively. A total of 65.1% and 82.1% of patients experienced complete response during the overall period in the ondansetron and palonosetron groups respectively [Table/Fig-5]. The difference in the response to antiemetic prophylaxis was statistically significant between the two groups for delayed (p-value=0.006) and overall phase (p-value=0.008).
Percentage distribution of patients based on the response to prophylactic antiemetic agents during acute, delayed and overall phases in the two groups.
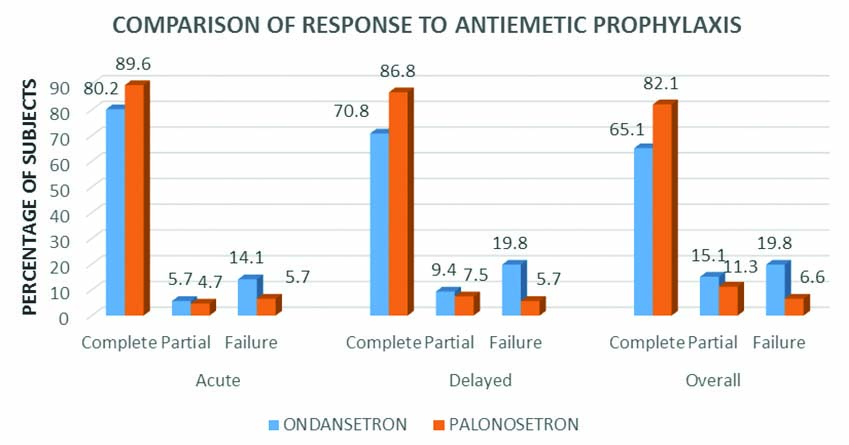
C. Adverse Effects
An 18 (17%) patients experienced headache, 9 (8.5%) had constipation, 2 (1.9%) had diarrhoea, 5 (4.7%) had abdominal pain and 3 (2.8%) had dry mouth during treatment in ondansetron group, whereas, in palonosetron group 10 (9.4%) patients experienced headache, 5 (4.7%) had constipation, nobody experienced diarrhoea, 1 (0.9%) had abdominal pain and 6 (5.7%) had dry mouth. There was no statistically significant difference between the two groups in relation to adverse effects and both groups tolerated the drugs well [Table/Fig-6].
Frequency (f) percentage (%) distribution of patients based on the adverse effects in the two groups.
| Sl. no | Adverse effects | Category of drugs |
|---|
| Ondansetron (n=106) | Palonosetron (n=106) |
|---|
| (f) | (%) | (f) | (%) |
|---|
| 1. | Headache | 18 | 17 | 10 | 9.4 |
| 2. | Constipation | 9 | 8.5 | 5 | 4.7 |
| 3. | Diarrhoea | 2 | 1.9 | 0 | 0 |
| 4. | Abdominal pain | 5 | 4.7 | 1 | 0.9 |
| 5. | Dry mouth | 3 | 2.8 | 6 | 5.7 |
Discussion
In this prospective observational study, we compared the prophylactic antiemetic effect of two 5HT3 receptor antagonists, ondansetron and palonosetron in patients receiving moderately or highly emetogenic cancer chemotherapy [11]. We selected the lowest effective single dose of ondansetron (8 mg) and palonosetron (0.25 mg) to determine the most effective prophylactic antiemetic agent for CINV [12]. The antiemetic effect was assessed similarly during acute phase, delayed phase and overall period in previous studies done by Gralla R et al., Aapro MS et al., and Kaushal J et al., [8,9,13]. There were no dropouts in the study. Comparison of demographic and baseline parameters of subjects included in ondansetron and palonosetron group showed no significant difference.
In acute phase, the difference in nausea free rates were numerically higher in palonosetron group in our study. The observation was similar to the previous study done by Kaushal J et al., [13]. The difference between ondansetron and palonosetron group was not statistically significant in both studies. The observation regarding nausea in delayed phase was similar to that of Kaushal J et al., [13]. In our study, palonosetron was significantly superior to ondansetron in preventing delayed nausea (p=0.027) but, there was no statistically significant difference between the two groups in the study done by Kaushal J et al., [13]. In both studies, during overall phase better control of nausea was observed in palonosetron group compared to ondansetron group with no statistically significant difference between the two groups.
In terms of control of vomiting during acute phase, even though numerically higher values were observed with palonosetron group, there was no statistically significant difference between the groups in our study. Results were similar to the study done by Kaushal J et al., with no statistically significant difference between the two groups [13]. Control of vomiting during delayed phase and overall phase in our study was significantly better with palonosetron unlike the other study where the difference was numerically higher without statistical significance [13].
The comparison of response to antiemetic prophylaxis by palonosetron and ondansetron during acute phase showed that complete response was achieved by 89.6% and 80.2% respectively. In the study done by Gralla R et al., with a sample size of 570 subjects, complete response during acute phase was achieved by 81% of subjects in palonosetron group compared to 68.6% in ondansetron group [8]. The difference observed between the two groups were statistically significant in the study done by Gralla R et al., (p=0.008) but no statistically significant difference was observed between the two groups in our study during acute phase. This might be due to a lesser sample size in the present study. In another study done by Aapro MS et al., [9]. It was observed that 59.2% of subjects in palonosetron group and 57% in ondansetron group showed complete response. The results were similar to the present study. Kaushal J et al., also observed similar response, where complete response in acute phase was 83.3% on palonosetron and 80% on ondansetron [13].
During delayed phase complete response was observed in 86.8% of subjects in palonosetron group and 70.8% in ondansetron group. The difference between the two groups was statistically significant. Complete response rates observed in study done by Gralla R et al., was 74.1% versus 55.1% in palonosetron and ondansetron group respectively (p=0.001) [8]. Even though the complete response rate was numerically higher in studies conducted by Aapro MS et al., (45.3% versus 38.9% in palonosetron and ondansetron group respectively) [9] and Kaushal J et al., (76.75 and 66.7% in palonosetron and ondansetron group respectively) there was no statistically significant difference between the two groups in those two studies [13].
Complete response of overall CINV for the palonosetron and ondansetron groups were 82.1% and 65.1% respectively in our study. In the study done by Gralla R et al., it was noted that palonosetron had complete response of 69.3% and ondansetron with 50.3% [8]. In both studies, the difference between the two groups were statistically significant. In the studies done by Aapro MS et al., (palonosetron versus ondansetron 40.8% versus 33%) and Kaushal J et al., (66.7% in palonosetron group and 46.7% in the ondansetron group) [9,13], it was found that complete response rates of palonosetron and ondansteron overall period were numerically higher but the difference between the two groups was not significant statistically. The results of the present study confirm the previous reports on superiority of palonosetron over ondansetron in reducing the incidence of CINV, especially in delayed CINV.
Similar studies on comparison on the effectiveness of palonosetron versus ondansetron were also done by Wenzell CM et al., Kovacs G et al., Mattiuzzi GN et al., and the results goes hand in hand with the present study [Table/Fig-7] [14-16].
Comparison of present study with similar previous studies [8,9,13-16].
| Author name | Reference number | Age group studied | Total no of subjects | Complete response | Final outcome |
|---|
| Arm | Acute | Delayed | Overall |
|---|
| Gralla R et al., | [8] | >18 years | 570 | Palonosetron | 81% | 74.1% | 69.3% | Palonosetron was significantly superior to ondansetron |
| Ondansetron | 68.6% | 55.1% | 50.3% |
| Aapro MS et al., | [9] | >18 years | 667 | Palonosetron | 59.2% | 45.3% | 40.8% | Palonosetron was as effective as ondansetron |
| Ondansetron | 57% | 38.9% | 33% |
| Kaushal J et al., | [13] | 25-60 years | 60 | Palonosetron | 83.3% | 76.7% | 66.7% | Palonosetron slightly better than ondansetron |
| Ondansetron | 80% | 66.7% | 46.7% |
| Wenzell CM et al., | [14] | 18-89 years | 80 | Palonosetron | 75% | 65% | 65% | Complete response rates was better with palonosetron but was not statistically significant |
| Ondansetron | 55% | 45% | 40% |
| Kovacs G_et al., | [15] | 0-17 years | 502 | Palonosetron | 95% | - | - | Palonosetron (20 μg/kg) non-inferior to ondansetron in acute phase |
| Ondansetron | 90% | - | - |
| Mattiuzzi GN et al., | [16] | >18 years | 97 | Palonosetron | - | - | 46% | Palonosetron given daily for four or five days significantly reduced CINV |
| Ondansetron | - | - | 34% |
| Present study | | 18-80 years | 212 | Palonosetron | 89.6% | 86.8% | 82.1% | Palonosetron is significantly superior to ondansetron |
| Ondansetron | 80.2% | 70.8% | 65.1% |
After overall assessment, it is seen that palonosetron group shows clinically better antiemetic efficacy than ondansetron group in preventing CINV and has significantly higher efficacy than ondansetron group in preventing delayed emesis. Palonosetron has shown superior results in controlling CINV in the present study. Palonosetron can be considered as a better choice for control of CINV, especially delayed CINV.
Limitation
The study has some limitations. Chemotherapy patients were assessed only in first cycle of chemotherapy. Secondly, no assessment was done for anticipatory and break through emesis. Moreover, this is a single institution based study. Results of the study need to be confirmed by further studies with larger sample size as multicenter trials.
Conclusion
Palonosetron is significantly more efficacious in preventing CINV, especially in delayed phase (24-120 hours) and overall period (0-120 hours). Though, palonosetron was found to be clinically more effective in acute phase, the statistical significance could not be obtained. Palonosetron can be considered as a better choice for control of CINV, especially delayed CINV.