Lichenoid Dysplasia–A Case Report with a Review of Differential Diagnosis
Kumud Mittal1, Mihir Jha2, Roopa S. Patil3, Shekhar Kapoor4
1 Reader, Department of Oral Medicine and Radiology, Sarabha Dental College and Hospital, Ludhiana, Punjab, India.
2 Senior Lecturer, Department of Paediatric and Preventive Dentistry, MGM Dental College and Hospital, Navi Mumbai, Maharashtra, India.
3 Postgraduate Student, Department of Oral Pathology and Microbiology, Bapuji Dental College and Hospital, Davangere, Karnataka, India.
4 Associate Professor and Head, Department of Oral Medicine and Radiology, Christian Dental College, CMC, Ludhiana, Punjab, India.
NAME, ADDRESS, E-MAIL ID OF THE CORRESPONDING AUTHOR: Dr. Mihir Jha, Senior Lecturer, Department of Paediatric and Preventive Dentistry, MGM Dental College and Hospital, Navi Mumbai, Maharashtra, India.
E-mail: doctormihirjha@gmail.com
Lichenoid Dysplasia (LD) is often regarded as lichen planus with dysplastic features, as it mimics lichen planus clinically and histologically. Although it has been confirmed that these two entities are entirely different with not so similar etiopathogenesis, yet the confusion still exists. The separation between the two is of utmost importance as each of them has their own prognosis and treatment plan. We report one such case, where a 51-year-old male with excessive burning sensation had similar clinical picture as that of lichen planus but was histologically diagnosed as LD.
Lichen planus, Lichenoid reaction, Squamous cell carcinoma
Case Report
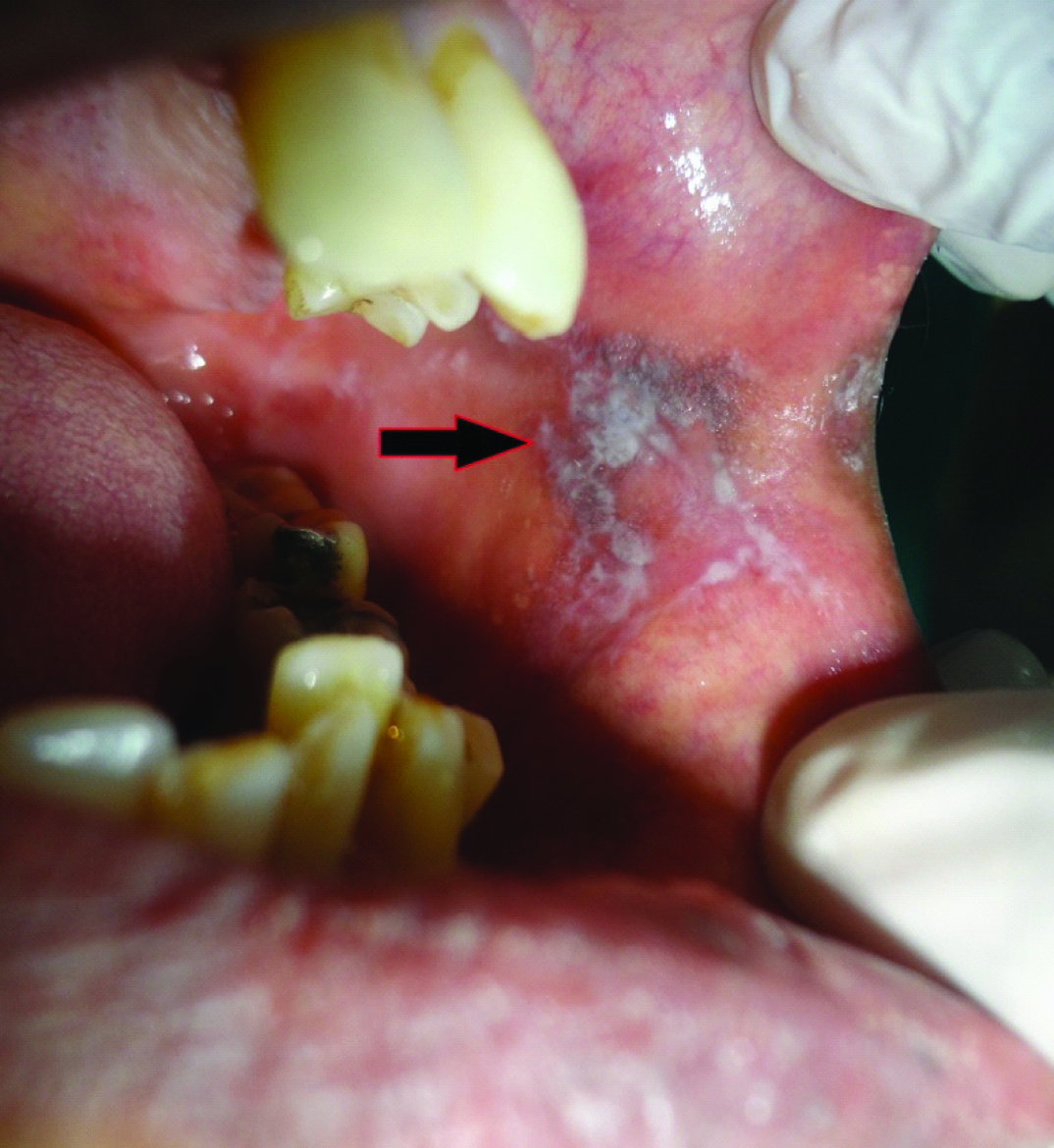
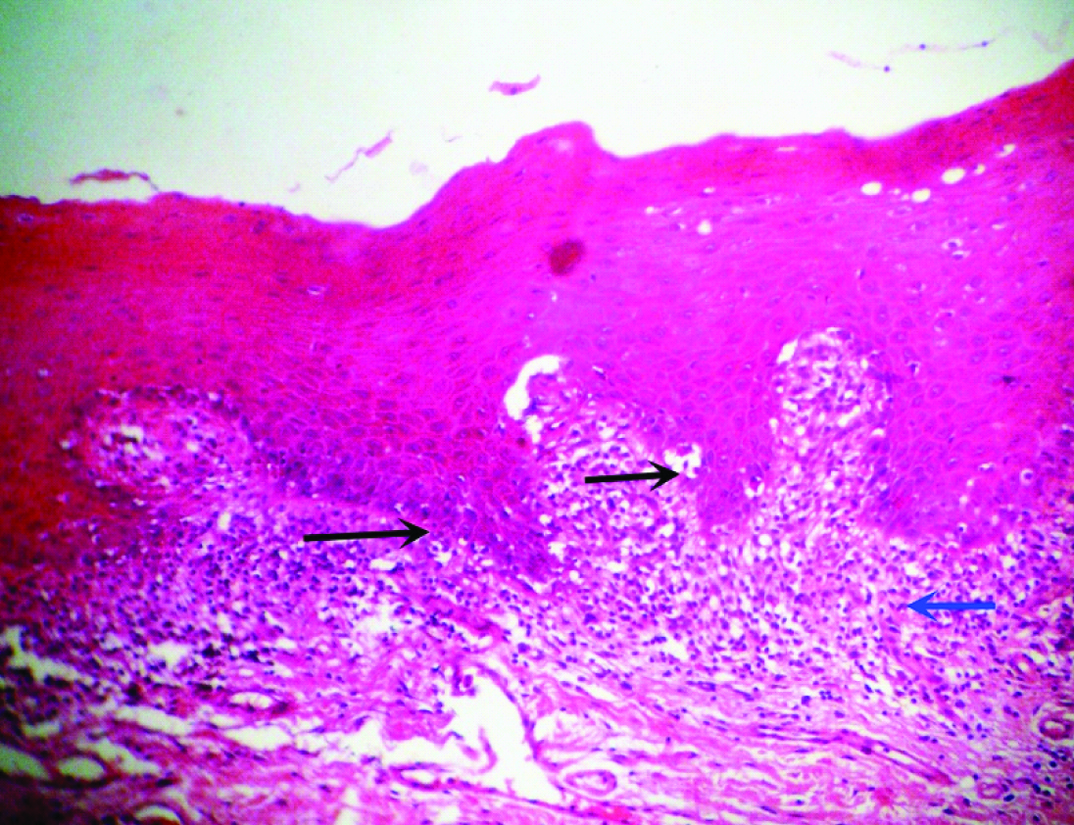
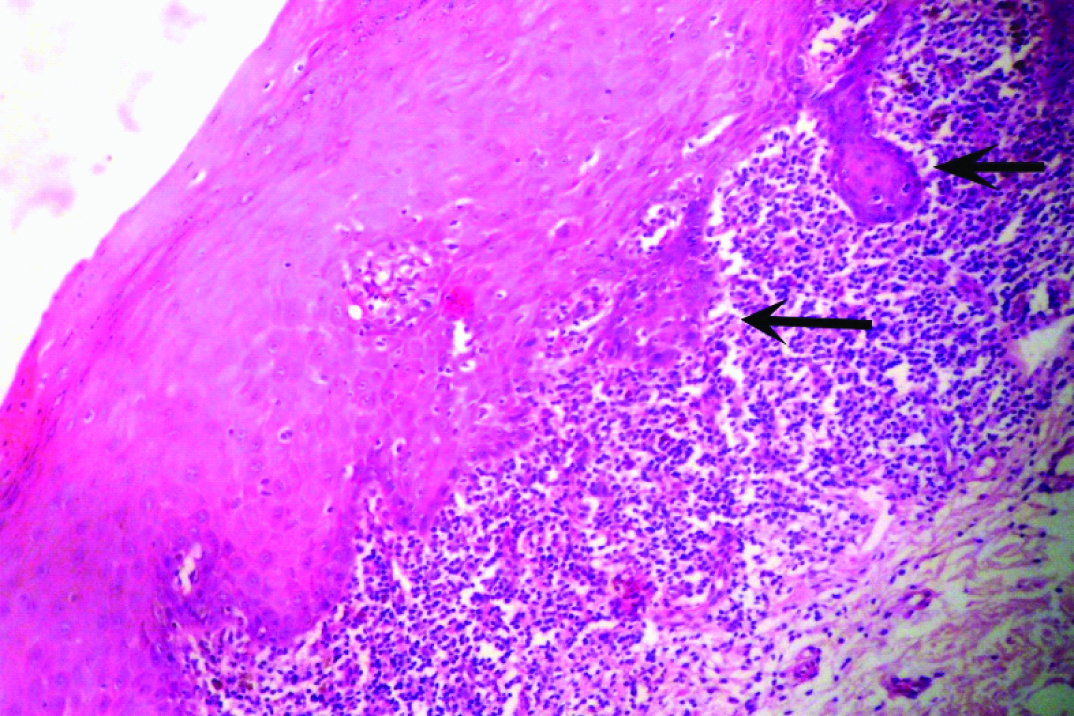
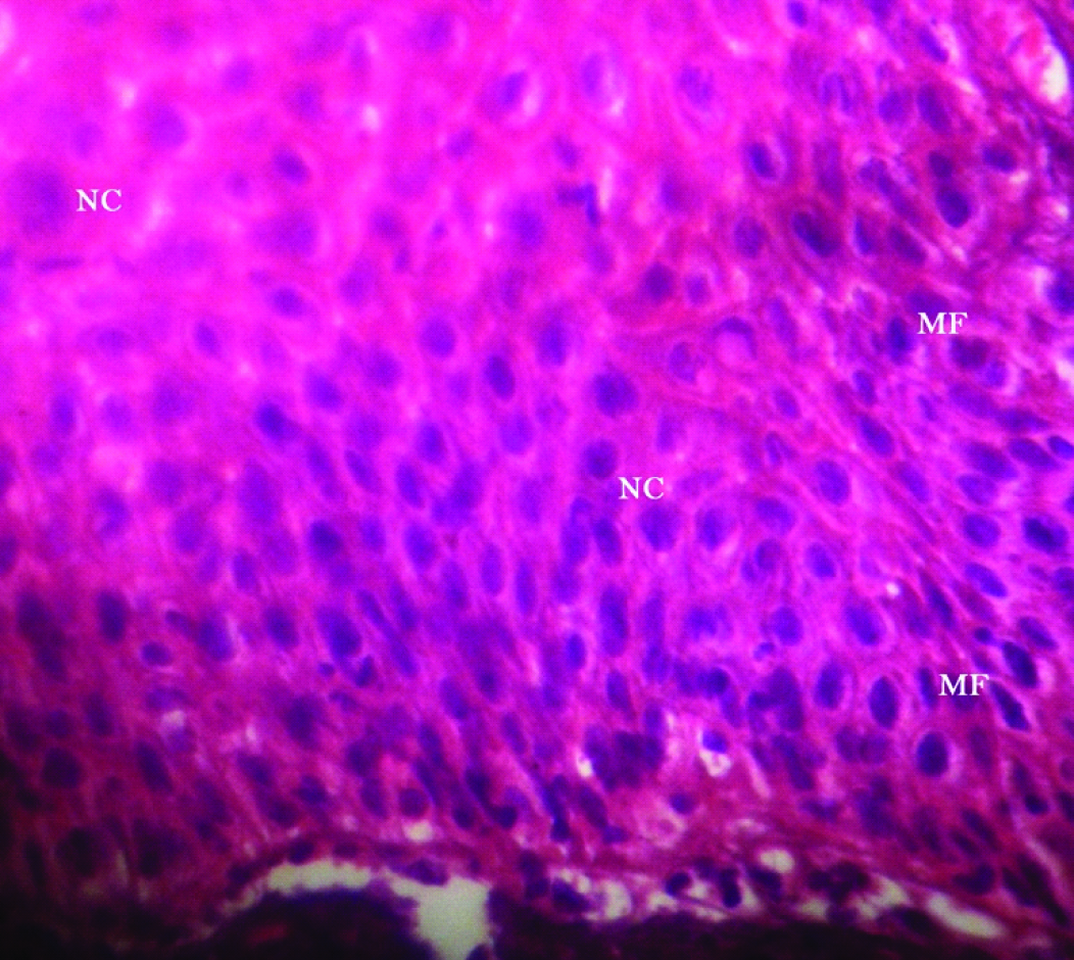
A 51-year-old male patient reported to the Dental Outpatient Department with a chief complaint of excessive burning sensation upon eating spicy food since two months. Since then, the severity of burning sensation has progressively increased. He got an amalgam restoration done in the tooth #37 around ten years back and did not change any tooth powder or paste recently. Medical history did not reveal any significant finding. Patient has been consuming beedi (1 packet/day) and chewable tobacco mixed with slaked lime for the past twelve years. Intraoral examination revealed grayish white lace like lesion on left buccal mucosa [Table/Fig-1]. As the patient did not notice any lesion before, so any change in the lesion could not be commented. The lesion had irregular margins with slightly raised surface and measured around 1.8x1.6 cm, extending from left commissure till premolar area. The oral mucosa elsewhere was clinically normal. Clinically, a provisional diagnosis of lichen planus and leukoplakia was made. After taking the patient’s consent, an excisional biopsy was performed and the patient was advised to discontinue tobacco usage in any form. Histopathological examination revealed hyperparakeratinized hyperplastic epithelium with saw tooth shaped rete ridges and juxta epithelial band of chronic inflammatory cells chiefly lymphocytes. Basal cell degeneration and Max-Joseph spaces were also evident [Table/Fig-2]. In addition, an area showing transformation of saw tooth shaped rete ridges into drop shaped rete ridges was seen [Table/Fig-3]. The epithelium in this area revealed dysplastic features like irregular stratification, increased nuclear cytoplasmic ratio, nuclear hyperchromatism and increased mitotic activity [Table/Fig-4]. After correlating the clinical findings with the histological features, a final diagnosis of lichenoid dysplasia was made. After five years of periodic follow up and postsurgical excision, no sign of recurrence was observed.
Intraoral photograph revealing grayish white lace like lesion on left buccal mucosa, measuring around 1.8x1.6 cm, extending from left commissure till premolar area.
Photomicrograph revealing hyperparakeratinized hyperplastic epithelium with saw tooth shaped rete ridges (black arrows) and juxta epithelial band of chronic inflammatory cells (blue arrow) (H and E, 40X).
Photomicrograph showing an area of transformation of saw tooth shaped rete ridges into drop shaped rete ridges (black arrows) (H and E, 40X).
Photomicrograph showing dysplastic features like irregular stratification increased Nuclear-Cytoplasmic ratio (NC), nuclear hyperchromatism and Increased Mitotic Activity (MF) (H and E, 40X).
Discussion
In the present case, the lesion diagnosed as lichen planus clinically came out to be LD histologically. The term LD was first used by Eisenberg E and Krutchkoff DJ to describe a lesion which shows dysplasia with lichenoid features [1]. In other words, this term is to be used when there are lichen planus like histological features in dysplastic epithelium. It does not imply dysplastic changes in lichen planus. It is neither a variant nor a transitional form of lichen planus. The pathogenesis of these two lesions is entirely different - in lichen planus the lichenoid infiltrate represents cell mediated immune response incited by different antigens whereas in LD, it represents immune surveillance mechanism against atypical epithelial cells [2]. Clinically these two appear similar, but LD is usually unilateral (as seen in our case report) and is more frequently seen on cancer prone sites like floor of the mouth, tongue, mandibular lingual alveolar ridge, soft palate, tonsillar pillar and uvula. Although the incidence of LD is far less than lichen planus, but when present its distinction from the latter is often difficult. The malignant potential of oral lichen planus is questioned in the recent past. Numerous studies have disclosed the fact that most of the previously reported cases of malignant transformation were either a Lichenoid Lesion (LL) or LD misdiagnosed as lichen planus. Thus LDs and LLs have propensity for transformation, but not the lichen planus and hence the differentiation between these comparably similar entities is very important [3-5]. Van der Waal I [6,7] classified oral lichenoid lesions (OLL) into following four types:
a) Amalgam restoration, topographically associated OLL;
b) Drug related OLL;
c) OLL in chronic Graft Versus Host Disease (GVHD); and
d) OLL unclassified (e.g., erythematous changes in gingiva without signs of “classic” lichen planus elsewhere in the oral cavity, or lesions that have a lichen planus like aspect but lack one or more characteristic clinical features like bilateral presentation).
In addition to these four categories, Kumar S et al., included erythema multiforme, lupus erythematosus and lichenoid dysplasia as these lesions are characterized by lichenoid tissue reaction [2]. Lichenoid reaction associated with amalgam or other dental restorations can be differentiated clinically on the basis of its unilateral presentation and its topographical relation with the amalgam restoration. Histologically, lymphoid follicle formation chiefly comprised of neutrophils and plasma cell is seen.
Drug related LL is considered as a different form of delayed hypersensitivity reaction, wherein drug or its metabolite acts as haptens. A history of drug usage in the recent past helps in making the clinical diagnosis. Histologically, the inflammatory infiltrate with predominant eosinophils is more widely spread deep into the connective tissue. Similarly in chronic GVHD, history of graft in patients with LL guides the diagnosis clinically. Histopathologically, subepithelial vesiculation, minimal lymphocytic infiltrate and perivascular cuffing of inflammatory cells distinguishes GVHD lesion from characteristic lichen planus.
At times, oral lesions in discoid lupus erythematosus reveal lichen planus like white striae. Clinically, such lesions are usually unilateral and have a predilection for vermillion area, palate and buccal mucosa. Histologically, a characteristic Periodic Acid Schiff (PAS) stained positive material is seen along the basement membrane and around the blood vessels. In addition, hyperkeratosis with plugging, perivascular infiltration and more deeply spread inflammatory infiltrate into the stroma are other significant differences [2,8]. In erythema multiforme (in absence of skin lesions), the histopathological resemblance with lichen planus can be ruled out clinically more efficiently. Lesions in erythema multiforme are more common in anterior part of the oral cavity; blood crusted ulceration on lips are almost exclusively seen in erythema multiforme. A definite history of exposure to known drugs inciting the hypersensitivity reaction or a recent herpes virus infection makes clinical correlation more valuable in clinching the diagnosis [9,10].
Kamath VB et al., suggested topical corticosteroids for management of lichen planus as well as LLs [11]. In case of LLs for which a distinct cause can be found warrants the removal of associated causative agent (like drug, amalgam restoration). In view of malignant transformation, biopsy is mandatory for LD and erosive and atrophic forms of lichen planus. Three month review following the treatment for the first year and biannually for the next two years is recommended. Repeated biopsies to be done when recurrence, change or spread is detected.
Conclusion
This case was clinically diagnosed as lichen planus but was confirmed to be LD histologically. The malignant potential of a lichen planus has been a matter of debate from ages, whereas LD is definitely premalignant, thus the distinction between the two entities is important. Moreover, an array of lesions with the term “lichenoid” exists, which often confuses the clinician, thus an attempt has been made to clearly guide the clinician to arrive at the final diagnosis.
[1]. Eisenberg E, Krutchkoff DJ, Lichenoid lesions of oral mucosa. Diagnostic criteria and there importance in the alleged relationship to oral cancerOral Surg Oral Med Oral Pathol 1992 73:699-704. [Google Scholar]
[2]. Kumar S, Hiremath S, Kale AD, Charantimath S, Oral lichenoid lesions: Clinico-pathological mimicry and its diagnostic implicationsJ Dent Res 2011 22(6):827-34. [Google Scholar]
[3]. Epstein JB, Wan LS, Gorksy M, Zhang L, Oral lichen planus: Progress in understanding its malignant potential and the implications for clinical managementOral Surg Oral Med Oral Pathol Oral Radiol Endol 2003 96:32-37. [Google Scholar]
[4]. Lodi G, Scully C, Carrozo M, Griffiths M, Sugarman PB, Thongprasom K, Current controversies in oral lichen planus. Report of an international consensus meeting part 2: Clinical management and malignant transformationOral Surg Oral Med Oral Pathol Oral Radiol Endod 2005 100:164-78. [Google Scholar]
[5]. Gonzalez-Molis MA, Scully C, Gil-Montoya JA, Oral lichen planus: Controversies surrounding malignant transformationOral Dis 2008 14:229-43. [Google Scholar]
[6]. van der Waal I, Oral lichen planus and oral lichenoid lesions; A critical appraisal with emphasis on the diagnostic aspectsMed Oral Patol Oral Cir Bucal 2009 14:E310-14. [Google Scholar]
[7]. van der Meij EH, van der Waal I, Lack of clinicopathological correlation in the diagnosis of oral lichen planus based on the presently available diagnostic criteria and suggestion for modificationsJ Oral Pathol Med 2003 32:507-12. [Google Scholar]
[8]. Orteu CH, Buchanan JA, Hutchison I, Leigh IM, Bull RM, Systemic lupus erythematosus presenting with oral mucosal lesions: Easily missed?Br J Dermatol 2001 144:1219-23. [Google Scholar]
[9]. Farthing PM, Bagan J, Scully C, Erythema multiformeOral Dis 2005 11:1-8. [Google Scholar]
[10]. Ayango L, Rogers RS 3rd, Oral manifestations of erythema multiformeDermatol Clin 2003 21:195-205. [Google Scholar]
[11]. Kamath VV, Setlur K, Yerlagudda K, Oral lichenoid lesions- a review and updateIndian J Dermatol 2015 60(1):102 [Google Scholar]