Pancreatic carcinoma accounts for a significant propotion of cancer related death toll in developing countries like India. Increase in the incidence has been linked to risk factors like lifestyle modification associated with increased alcohol consumption and rapid urbanisation [1]. Dhir V reported an incidence of 0.5-2.4 per 1,00,000 men and 0.2-1.8 per 1,00,000 women in India, with higher rates seen in the male urban populations of western and northern India [2]. Balakrishnan V et al., recently conducted a multicentre study to assess chronic pancreatitis in 1086 subjects which showed an incidence rate of about 4% for pancreatic tumours [3]. Only 16% of patients initially present with a disease completely confined to the pancreas [4,5], with about 85%-90% having surgically unresectable tumours at the time of diagnosis [4-6]. Surgical resection offers the only chance for cure and imaging plays a crucial role in the early diagnosis of the condition; the various imaging modality currently used in the diagnosis and preoperative staging of carcinoma pancreas includes; Ultrasonography (USG), contrast-enhanced Computed Tomography (CT), Magnetic Resonance Imaging (MRI), magnetic resonance cholangiopancreatography and endoscopic ultrasound. Contrast enhanced computed tomography is the imaging modality of choice for diagnosis and preoperative staging of the condition.
Preoperatively pancreatic carcinoma can be categorized into resectable, unresectable and borderline resectable tumours. Bipat S et al., in a meta-analysis, found a sensitivity of 81% and specificity of 82%, for determining resectability [7]. Borderline resectable tumours has been defined by National Comprehensive Cancer Network as tumours that display: (a) venous involvement of the superior mesenteric vein-portal vein confluence with possibility of vascular reconstruction; (b) encasement of gastroduodenal artery up to hepatic artery or hepatic artery involvement with no celiac extension; or (c) tumour abutting superior mesenteric artery less than 1800 [8]. MRI has shown results like CT and is reserved for those patients on whom CT cannot be performed. Newer sequences such as diffusion weighted imaging has shown fruitful results in the evaluation of pancreatic lesion using quantitative analysis by calculating mean ADC values [9].
CT and MRI are excellent modalities in assessment of perineural invasion associated with pancreatic carcinoma. On CT perineural invasion should be suspected when: (1) peripancreatic fat plane is replaced by soft tissue; (2) loss of fat plane around Superior Mesenteric Artery (SMA) or superior mesenteric vein; (3) loss of fat plane around celiac trunk and (4) loss of fat plane around splenic vein [10].
On MRI perineural involvement is graded on assessing the regions posterior and medial to the pancreatic head, posterior to body of the pancreas and the involvement of major vessels adjacent to the tumour, including the SMV and/or portal vein, SMA, celiac axis artery, common and/or proper hepatic artery, and splenic artery as; NV0- signal intensity of fat adjacent to the lesion shows no change, NV1- signal intensity suggestive of fat standing, NV2-mass larger than 1 cm adjacent to the lesion [10].
The purpose of this study was to assess the accuracy of multidetector CT in detection and staging of pancreatic carcinoma in a tertiary referral centre in Kerala. The primary objective of the study was to compare the staging of pancreatic carcinoma by MDCT with surgery in a preoperative setting.
Materials and Methods
This cross-sectional observational study was conducted between November 2014 and October 2016. The study was performed after obtaining approval from Thesis Protocol Review Committee (Scientific, Ethical and Financial), Amrita Institute of Medical Sciences. Based on validity parameter- sensitivity of multidetector CT with respect to surgery [11]; with 95% confidence and 20% allowable error, a minimum sample size of 10 was obtained. Twenty-five patients (12 men, 13 women; mean age, 54.2 years; range, 17-72 years) were referred to the department with a known or suspected diagnosis of pancreatic carcinoma and underwent contrast enhanced CT scan, of which, 7 (28%) were considered inoperable because of metastasis [Table/Fig-1]. Patients with history of previous pancreatic surgery, patients post- neo adjuvant chemotherapy and patients with contraindication to CT imaging were excluded from the study.
Demographic details of the patients.
| n | Minimum(yrs.) | Maximum(yrs.) | Mean(yrs.) | Median(yrs.) | Std.Deviation |
|---|
| Age | 25 | 17 | 72 | 54.28 | 53 | 12.047 |
| |
| Age groups | Number of patients (n-25) | % of patients |
| <40 years | 1 | 4% |
| 40-50 years | 6 | 24% |
| 50-60 years | 10 | 40% |
| 60-70 years | 4 | 16% |
| 70-80 years | 4 | 16% |
| Gender | Frequency (n-25) | Percent |
| Female | 13 | 52.0 |
| Male | 12 | 48.0 |
| Total | 25 | 100.0 |
MDCT technique: A total of 25 patients with suspected pancreatic pathology were evaluated using three different CT machines due to high patient load on the department, a 16 slice multi detector CT (Somatom, E-motion 16, Siemens Healthcare; Germany), 64 slice multi detector CT (Somatom, Sensation Cardiac, Siemens Healthcare; Germany) and 256 slice multi detector CT (Brilliance iCT; Philips Healthcare, Cleveland, OH). Cases were randomly allocated to the three systems. Contrast enhanced CT was performed after a four hour fasting phase. Patients were examined in supine position, and each patient was instructed to remain stable, and not move with suspended breathing during the CT acquisition [Table/Fig-2].
| Siemens 16 slice (Somatom, Emotion) | Siemens 64 slice (Somatom, Sensation Cardiac) | Philips Brilliance iCT 256 Slice |
|---|
| Collimation | Arterial: 16x0.6 mm Venous: 24x1.2 cm | Arterial: 64x0.6 mm Venous: 24x1.2 cm | Arterial: 256x0.625 mm Venous: 256x0.625 mm |
| Pitch | 1.2 | 1.5 | 0.914 |
| Rotation time | 0.5 sec | 0.5 sec | 0.5 sec |
| kV | 120 | 120 | 100 |
| mA | 120 | 120 | 151 |
| Bolus tracking | No | No | No |
Non-ionic contrast material Omnipaque (Iohexol 350, General Electric Healthcare Little Chalfont, United Kingdom) was used with an iodine concentration of 350 mg/ml. In patients with high serum creatinine levels or patients with eGFR ranging between 30-60 ml/min/1.73m2, Visipaque (iodixanol 320 General Electric healthcares, Little Chalfont, United Kingdom) with an iodine concentration of 320 mg/ml was used.
A plain scan was taken covering the upper abdomen including the pancreas. Oral contrast agent was given for opacification of the gastrointestinal tract. Gastrografin/Gastrolek (Diatrizoate Meglumine and Diatrizoate Sodium 76%) was given before performing contrast enhanced CT (40 ml of contrast in one litre of water). Scan was performed 45 minutes after administration of oral contrast. Contrast was injected using a pressure injector through an 18-gauge cannula sited in an upper limb vein. Patients underwent a dual phase enhanced CT scan with the pancreatic parenchymal phase acquired at 35 seconds and portal venous phase acquired at 70 seconds [Table/Fig-3]. Lu DS et al., and Boland GW, showed that when using a dual phase scanning technique with pancreatic phase at 40 seconds and venous phase at 70 seconds, the pancreatic tumour to pancreatic parenchymal enhancement difference was significant and the surrounding vascular structures were adequately enhanced [12,13].
Contrast enhanced CT axial images depicts the importance of pancreatic protocol, image A shows pancreatic phase with pancreatic lesion “T” well visualized, which cannot be clearly made out in Image B which is acquired in venous phase.
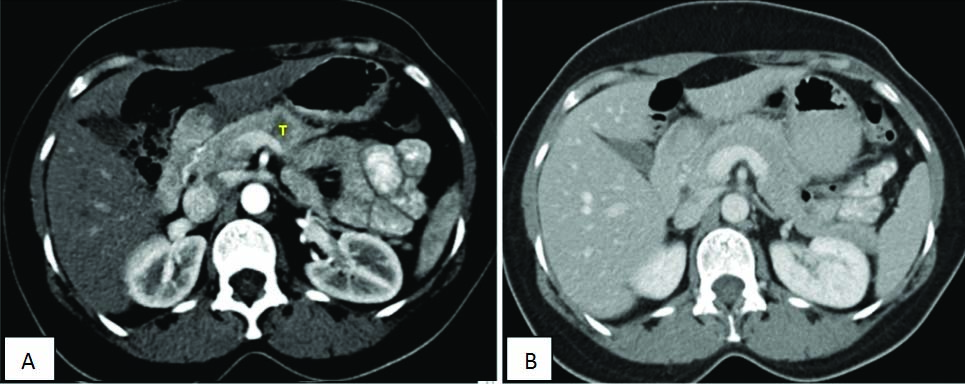
Image analysis: Multidetector computerized tomography allows assessment of the pancreas and its surrounding structures using thin sections. We used axial, multiplanar reconstruction and curved reformatted images during image interpretation.
The following data was recorded: 1) Location of the lesion: head, body, tail, size of the lesion; 2) Density: solid, cystic, 3) Enhancement pattern; 4) Pancreatic duct, Common bile duct, Intrahepatic biliary radical: dilatation/not dilated; 5) Regional lymph node involvement: anterior, posterior, superior and inferior groups; 6) Loco-regional involvement (Peripancreatic tissue, Bile duct, Stomach, Spleen): present/absent; 7) Metastasis (Liver/Peritoneal): present/absent; 8) Ascites: present/absent; 9) Vascular involvement: present/absent {Arterial- coeliac artery, superior mesenteric artery, common hepatic artery and gastroduodenal artery. Venous involvement- portal vein, superior mesenteric vein and inferior vena cava}.
Vascular involvement was assessed and graded based on Lu DS et al., into five grades: Grade 0-No contiguity of tumour to vessel; Grade 1- Tumour contiguous to less than one quarter circumference (loss of fat plane); Grade 2- involvement between one-quarter and one- half circumference (less than 1800); Grade 3-involvement between one-half and three-quarters circumference (more than 1800); Grade 4- greater than three-quarters circumferential involvement (occlusion) [12].
Once the data was recorded the tumour was staged as either: 1) Resectable; 2) Unresectable; 3) Borderline resectable. These findings were finally correlated with surgical outcome to assess accuracy in predicting respectability and vascular involvement [Table/Fig-4].
Criteria for resectability.
| Resectable Pancreatic tumours |
| 1 No distant metastases; |
| 2 No extension to the SMA, normal fat plant between the tumour and SMA; |
| 3 No extension to the coeliac axis or hepatic artery; |
| 4 Patent SMV/PV. |
| Unresectable pancreatic tumours |
| 1 Encased SMA (>180 degree); |
| 2 Encased HA with no option for reconstruction; |
| 3 Occluded SMV/PV with no option for reconstruction. |
| Borderline resectable |
| 1 Tumour abutment ≤180 degree of the circumference of the SMA; |
| 2 Short-segment encasement/abutment of the CHA (typically at the GDA origin); |
| 3 Short-segment occlusion of the SMV/PV with suitable vessel above and below. |
Statistical Analysis
Validity parameters namely sensitivity, specificity, accuracy and positive predictive/NPV was computed for MDCT with respect to surgery. Numerical variables were expressed as mean and standard deviations and the categorical variables were expressed as frequency and percentages. To compare the outcome of different methods McNemar test was applied. To compare the mean differences of numerical variables between different methods Wilcoxon Signed rank test was applied. To assess the association between categorical variables Fishers exact test was applied. All statistical analysis was done using IBM SPSS version 20.0.
Results
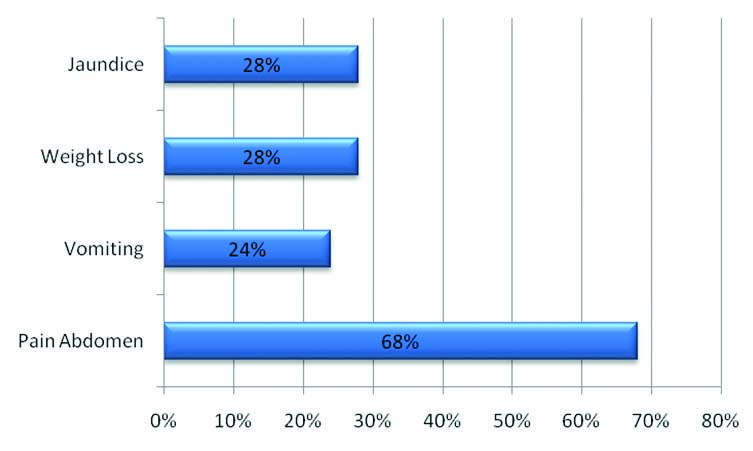
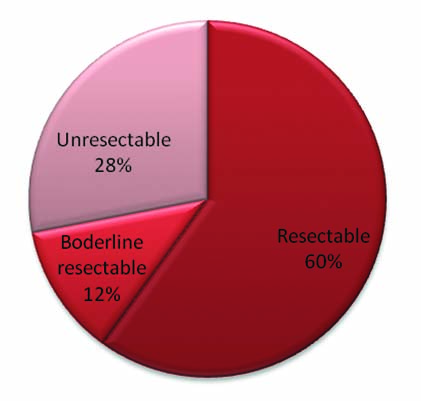
In our study of the 25 patients who were evaluated, 52% (13) were female patients and 48% (12) were male with a mean age of 54.2 and the median age of 53 years [Table/Fig-1]. Of the 25 patients, 17 complained of abdominal pain (68%), 6 of vomiting (24%), 7 of weight loss (28%) and 7 of yellowish discolouration (28%) [Table/Fig-5]. Ten (40%) patient clinically presented with a previous history of chronic pancreatitis and 15 (60%) presented with no previous history of pancreatitis. Of the 25 lesions, which were evaluated, 64% of the pancreatic tumours were in the head, 24% in the body of pancreas and 8% in the tail region. Of the 25 cases assessed, 12 (48%) cases showed both pancreatic duct and common bile duct dilatation, 7 (28%) showed isolated pancreatic duct dilatation, 2 (8%) showed isolated common bile duct dilatation and 4 (16%) showed no ducts to be dilated [Table/Fig-6]. A total of 68% (17) lesions were solid, 24% lesions had both solid and cystic elements and 8% were purely cystic appearing on CT scan. Of the 25 lesions, 13 (52%) lesions measured in a range of 20-40 mm, 6 (24%) were less than 20 mm and 6 (24%) more than 40 mm. On CT assessment, 15 (60%) cases were classified as resectable tumours, 3 (12%) as borderline resectable and 7 (28%) as unresectable tumours [Table/Fig-7].
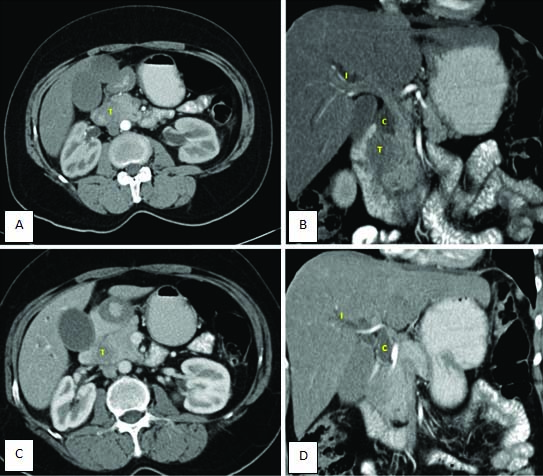
Contrast enhanced CT, Images a,c): Axial sections and images b,d): reformatted coronal images, shows pancreatic lesion “T” well visualized in pancreatic head with isolated common bile duct dilatation; “c” and intrahepatic biliary radicle dilatation; “I”. The lesion shows loss of fat plane with duodenum.
Distribution of tumour based on respectability.
CT showed a sensitivity of 82.3% with a low specificity of 87.5%, attributable to three patients who were diagnosed as unresectable on preoperative CT, turned out to be borderline resectable on table. The Positive Predictive Value (PPV) for diagnosing resectablity was 93.3% and NPV was 70%. On assessing vascular involvement CT showed a sensitivity of 100% with a specificity of 93.3%, the PPV was 75% and NPV was 100% [Table/Fig-8]. Accuracy of CT in comparison to surgery was 94.4% [Table/Fig-9].
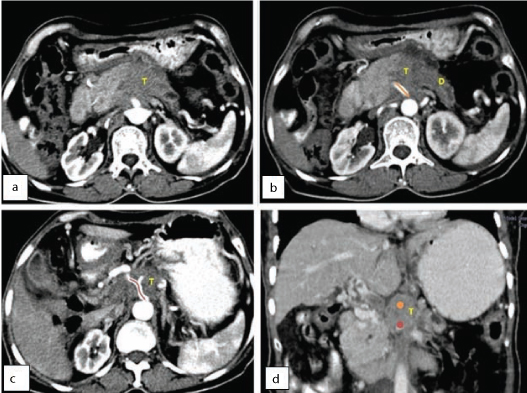
Contrast enhanced CT: a-c) Axial sections and; d) Reformatted coronal images, shows a hypoattenuating pancreatic mass lesion (T) in the region of body of pancreas. The lesion is seen involving major arteries like celiac artery (marked in orange) and superior mesenteric artery (marked in red). Pancreatic duct appears dilated (d).
MDCT in assessing respectability.
| CT | Surgery | p-value |
|---|
| Yes | No |
|---|
| n=17 | % | n=8 | % |
|---|
| Yes (15) | 14 | 82.3 | 1 | 12.5 | 0.625 |
| No (10) | 3 | 17.7 | 7 | 87.5 |
Validity parameters like sensitivity, specificity, accuracy and positive predictive/NPV were obtained by using McNemar test.
Of the 25 patients under evaluation, 15 (60%) cases had pancreatic adenocarcinoma, 3 (12%) serous neoplasm, 1 (4%) intraductal papillary mucinous neoplasm, 1 (4%) neuroendocrine tumour and 5 (3 mass forming pancreatitis, 1 medullary carcinoma, 1 poorly differentiated tumour (20%).
Discussion
Pancreatic carcinoma is among the deadliest malignancies with an increase in incidence rate in developing country like ours especially due to lifestyle changes. Surgical resection offers the only chance for cure and imaging plays a crucial role in the early diagnosis of the condition. Various imaging techniques are available like ultrasound, CT, MRI and PET scan.
The accurate determination of tumour resectability is the most important contribution of preoperative staging. Multidetector CT is an excellent modality for both detection and characterization of pancreatic tumours. Pancreatic tumours are categorised into resectable, unresectable and borderline tumour based on CT findings. Tumours are considered unresectable in presence of metastatic disease or local vascular invasion. This information is important for both the radiologist and the surgeon and further management plan.
In our study, pancreatic neoplasms were more common in females (52%) than males (48%). Our findings lie in contrast to Jemal A et al., who stated that the age-adjusted incidence rate of pancreatic neoplasm is greater in men than in women [14]. The age group most affected was between 50-60 years (40%) with a median age of 53 years. Manak E et al., found a mean age of 64.7, which was almost similar to our observations [15].
Clinically, 68% of patients complained of pain which was like Takhar et al., who found pain as the most common clinical complain and Chang NC et al., who stated that abdominal pain was present in 80%-85% of patients with locally advanced or advanced disease [16,17]. Jaundice was the second most common complain seen in about 28% of cases. 10% of our patients who developed pancreatic tumours had a clinical history of chronic pancreatitis and was consistent with Raimondi S et al., who stated a relative risk is 13.3% of patients developing pancreatic carcinoma with history of chronic pancreatitis [18].
In our study, 64% of the pancreatic tumours were in the head, 24% in the body of pancreas and 8% in the tail region. Freeny PC et al., in his study reported similar occurrences, being 62% in head, 26% in body and 12% in tail region [19].
In our study, 18 (72%) patients showed pancreatic duct dilatation,14 (56%) dilated common bile duct and biliary radicle dilatation in 11 (44%) patients and it was consistent with Freeny PC et al., findings who reported 68% of his cases showed pancreatic duct dilatation and 58% showed biliary duct dilatation [19]. Double duct sign was seen in 12 (48%), isolated pancreatic duct in 7 (28%), isolated CBD duct in 2 (8%) and no duct dilatation in 4 (16%). Freeny PC et al., found that 13% had double duct sign, 47% had only pancreatic duct dilatation, 8% had isolated biliary duct dilatation, and 32% had no evidence of pancreatic or biliary duct dilatation on CT scans [19].
Of the 25 patient included in our study, 15 (60%) pancreatic lesions were diagnosed as resectable, 3 (12%) were diagnosed as borderline resectable and 7 (28%) lesions were described as unresectable because six out of the seven lesion had advanced condition with liver metastasis and one patient was diagnosed of mass forming pancreatic mass on CT guided biopsy and was treated conservatively.
On assessing resectablility, CT showed a sensitivity of 82.3% and a specificity of 87.5% with a PPV of 93.3% and a NPV of 70%. The specificity was slightly lower as three patients who were considered unresectable due to superior mesenteric vein-portal vein involvement were found to be borderline resectable cases when taken up for palliative surgery and could undergo successful resection with vascular reconstruction. One false positive case was recorded as the patient on surgery was found to have an advanced growth with portal vein involvement requiring a long segment resection and hence a palliative gastro-jejunostomy surgery was performed. CT showed an accuracy of 84%.
Olivié D et al., reported a PPV of 82.6% [11]. A study by Bipat S et al., found a sensitivity of 81% and specificity of 82%, respectively in determining resectability [7]. Phoa SS et al., reported a NPV of 57% [20]. Our findings were almost consistent with the other studies.
Three (12%) cases in the study were classified as borderline resectable pancreatic tumours and on surgery these patients underwent tumour resection by Whipples procedure with vascular reconstruction. We believe MDCT can precisely categorise patients who can benefit from a complex vascular reconstruction surgery.
As vascular involvement plays a crucial role in deciding resectability, we compared the major vessel involvement on CT and surgery to assess the statistical significance. Of the 18 patients who underwent surgery, CT showed a sensitivity of 100% and a specificity of 93.3% with PPV of 75%, NPV of 100% and accuracy rate of 94.4%, using vascular involvement of more than 180 degree as criteria for unresectable tumours. Lu DS et al., reported a PPV of 95%, and a NPV of 93% with a sensitivity of 84% and specificity of 98% [12]. Phoa SS et al., found a sensitivity and specificity of 60% and 90% respectively [21].
On assessing arterial and venous involvement separately, CT showed sensitivity 100%, specificity 93.3%, PPV 75%, NPV 100.0% and accuracy rate of 94.4% in assessing venous involvement and for arterial involvement specificity of 94.4%, NPV 100.0% and an accuracy rate of 94.4%. Warshaw AL et al., showed PPV of 55% for venous invasion and 94% for arterial invasion and NPV of 95% for venous invasion and 94% for arterial invasion [22].
On assessing peripancreatic involvement of patients using obliteration of fat planes as criteria, CT showed sensitivity 100%, specificity 63.63%, PPV 60%, NPV 100% and an accuracy of 76.47% when assessing involvement of duodenum, bile duct, peripancreatic tissues. On assessing direct involvement of surrounding structures like stomach, spleen and colon, CT showed sensitivity 100%, specificity 92.85%, PPV 75%, NPV 100% and accuracy of 94.11% in assessing infiltration seen in 3 (17%) of patient. Freeny PC et al., in his study found local extension in 68% of patients and direct extension of tumour into adjacent organ in 42% of the patients [19].
Liver metastasis was seen in 6 (24%) out of 25 patients and it was confirmed with USG/CT guided biopsy. Murfitt J et al., stated that metastasis to the liver occurs in approximately 17%–55% of the patients and our findings agreed with it [23].
Limitation
Our study presents with the limitation of small sample size as many patients present with an advanced condition and offered palliative treatment as laparotomy in these cases are not suggested.
Conclusion
MDCT is a useful tool for diagnosis and staging of pancreatic carcinoma. It carries a high sensitivity and specificity for detection of vascular invasion which can be of great aid for preoperative planning.
Validity parameters like sensitivity, specificity, accuracy and positive predictive/NPV were obtained by using McNemar test.