Introduction
Multiple Sclerosis (MS), a disease of autoimmunity and inflammation, is characterized by deterioration of the myelin sheath that protects the nerve fibers. The high levels of neutrophils in serum may be related to the chronic inflammation and caused by other triggers such as infections that have been associated with relapses in MS.
Aim
To investigate the value of Neutrophil/Lymphocyte Ratio (NLR) as a possible marker and relationship with Depression, Anxiety and Stress (DAS) score in MS patients.
Materials and Methods
A total of 60 MS patients and 60 age and sex matched healthy controls were recruited for the present study. We measured DAS score, NLR, calcium, phosphate, magnesium, chloride, alkaline phosphatase, albumin in serum levels in MS patients and in healthy controls.
Results
The mean age was not significantly different in both case and control groups. The case and control groups were similar in terms of sex; however, the majority of the MS group was female. The NLR values of MS patients were significantly higher than those of the healthy controls (p=<0.001). The NLR values were also significantly (p<0.001) correlated with stress score.
Conclusion
NLR could be considered as a quick, cheap, easily measurable and inflammatory marker for assessment of inflammation in MS patients. The role of NLR in MS must be explored further.
Introduction
MS is a complicated chronic inflammatory demyelinating disease of the brain [1]. The exact aetiology of MS is still unknown, however immunologic, infectious, genetic and environmental factors are considered to play an important role in causing this disease [2].
The high levels of neutrophils in serum may be related to the chronic inflammation and caused by other triggers such as infections that have been associated with relapses in MS [3]. White blood cell count and its subtypes are markers of systemic inflammation [3,4]. NLR, calculated by dividing neutrophils count over lymphocytes count, has been linked to many diseases as a biomarker or a predictor of prognosis such as schizophrenia [5], resistant hypertension [6], myocardial infarction [7], chronic obstructive lung disease [8], pancreatic cancer [9], and MS [10]. In MS patients, increased serum levels of some inflammatory markers are detectable [11,12].
MS causes physical, cognitive, and emotional disorders. Depression and anxiety are common in MS patients [13]. Pathogenesis of mood disorders in MS is not well understood but seems multifactorial combining direct inflammatory effect in selected CNS tissues with neurophysiological disturbance of emotion [14], possibility of being MS drug induced [15], and it could be patients’ response towards physical disability as measured in expanded disability status scale [16]. It is also not clear whether depression and anxiety share same mechanisms; depression being more severe or they actually have different pathogenesis. Coexisting emotional disorder may limit treatment compliance and rehabilitation. The hypothesis of this study was that the relationship between Depression, Anxiety and Stress (DAS) score and inflammation using NLR can be used as a predictive and reliable marker.
Therefore, aim of the study was the measurement of NLR, as well as an exploratory analysis of other clinical chemistry parameters closely related to NLR, namely serum levels of calcium, phosphate, magnesium, chloride, Alkaline Phosphatase (AlkPhos) and Alanine Aminotransferase (ALT) and Aspartate Aminotransferase (AST) in MS patients and age and sex matched healthy controls in the study.
Materials and Methods
In this study, 60 MS patients were evaluated for their eligibility for inclusion as complete clinical data containing laboratory records, blood count (CBC) and DAS score. Sixty gender and age matched healthy subjects without any risk factors or chronic diseases were included as controls. Written informed consent was obtained from each of the subject. This study was approved by the Ethics Committee of College of Medicine, King Saud University, Riyadh, Saudi Arabia. The study was carried out during the time period from October 2015 until April 2016.
Biomarker Assessment, Evaluation of NLR and Other Inflammatory Markers
After collection of all venous blood samples in the dipotassium-Ethylenediaminetetraacetic Acid (EDTA) tubes (Beckman Coulter UniCel DxH 800), blood cell analyser was used to measure CBC within one hour after venipuncture. This duration is standard for our university hospital laboratory and helps to prevent EDTA induced swelling. Dimension RxL Max (Siemens) chemistry analyser was used to measure all biochemistry variables.
Neutrophils and lymphocytes were extracted from the blood samples. NLR was calculated as the ratio of neutrophil and lymphocyte count obtained from the same blood sample.
DAS Scale-21 (DASS-21)
Arabic version of this instrument [17] was used to assess DAS of MS patients. This tool has successfully been used in a previous study and is recommended to evaluate neurological disorders which appear in psycho-neuro formats [18]. This scale consists of 21 questions. Each of three subscales contains seven questions, the scores of which are obtained by summing up the scores of related questions. Evidences from the studies confirm the preliminary reliability and preliminary construct validity of the Arabic version of DASS-21 [19].
Statistical Analysis
All statistical analyses were performed using the Statistical Package for the Social Sciences for Windows version #20.0 (SPSS Inc., Chicago, IL, USA). The Kolmogorov Smirnov test was used to determine normal distribution of data. Continuous variables were expressed as mean {standard deviation (SD)} according to distribution state. Categorical variables were expressed as numbers and percentage. Student’s t-test or Mann–Whitney U test was used to compare the two independent groups according to distribution state. Pearson’s correlation test was done to test for significant correlations for (NLR and other laboratory values. Two-tailed p-values<0.05 were considered statistically significant.
Results
Among 60 MS patients, 12 (20%) were males and 48 (80%) were females. The mean±SD age was 36.44±9.567 years. The control group consisted of 20 (33.33%) males and 40 (66.67%) females. The mean age±SD was 38.21±14.63 years. The mean age was not significantly different in both groups. The case and control groups were similar in terms of numbers; however, females were in majority as compared to males in both groups. Among MS patients, females were higher in number compared to males. The demographic characteristics of the patients are summarized in [Table/Fig-1].
Demographic data of the case and control groups.
| Variable | Cases | Controls |
|---|
| Mean±S.D. | Mean±S.D. |
|---|
| Age | 36.44±9.56 | 38.21±14.63 |
| Gender | 48 Females/12 Males | 40 Females/20 Males |
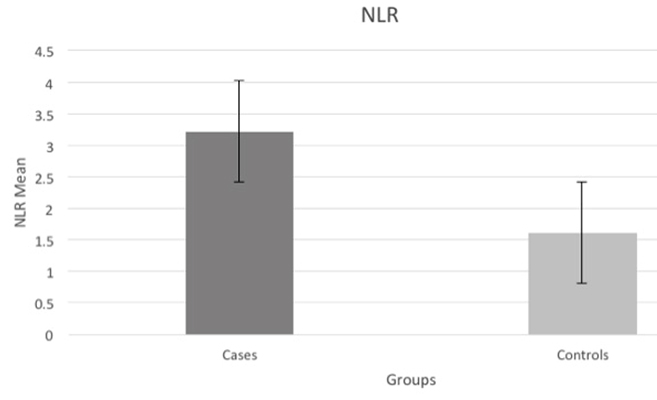
The mean NLR±SD was significantly higher in the case group 3.21± 3.60 compared to that of control group 1.61±2.91 (p=0.001) [Table/Fig-2] in parallel with the increase in neutrophils and the decline in lymphocytes in cases compared to controls.
Mean NLR values of the groups;
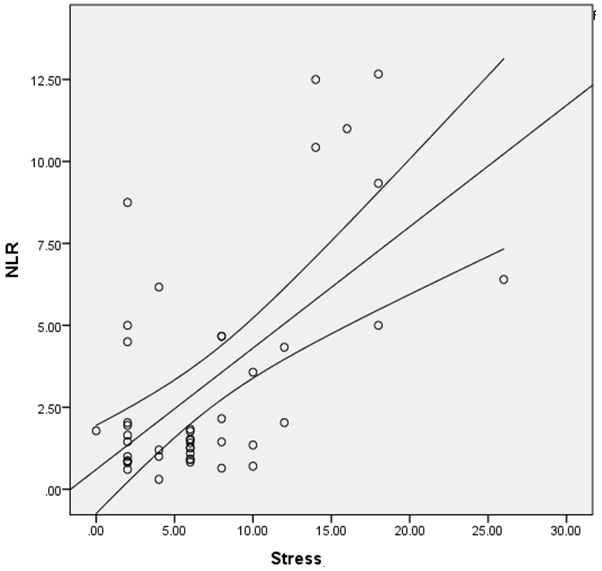
In the evaluation of patients with MS, NLR showed positive correlation with stress score (r=0.372, p<0.001) [Table/Fig-3]. Otherwise, there were no significant correlations between other inflammatory markers in the case group [Table/Fig-4].
The association between NLR and stress among MS patients.
Correlation of significant variables among cases.
| Variable | NLR | Hct | ALT | Na+ |
|---|
| Stress | <0.001** | .949 | .551 | .004* |
| Anxiety | .066 | .999 | .756 | .179 |
| Depression | .800 | .357 | .206 | .778 |
* Pearson’s correlation test
*. Correlation is significant at the 0.05 level (two-tailed).
**. Correlation is heighly significant at the 0.001 level (two-tailed).
Exploratory analysis of clinical chemistry parameters related to NLR in the case and control groups:
When the case and control groups were compared with each other, a statistically significant difference was found between the groups in terms of RBC, NLR, glucose and Ca+ (for all parameters p<0.05). NLR, Na+ and Cl- were higher in the case group. On the other hand, Ca+, glucose and AlkPhos were lower in this group compared with controls [Table/Fig-5,6].
Laboratory data of the case and control groups.
| Variable(μ/l) | Cases | Controls | p-value |
|---|
| Mean±S.D. | Mean±S.D. |
|---|
| WBC | 6.34±3.06 | 6.78±2.09 | 0.362 |
| RBC | 4.53±0.52 | 4.77±0.63 | 0.020 |
| Hgb | 124.35±17.94 | 128.33±23.82 | 0.290 |
| Hct | 37.52±4.86 | 39.33±6.54 | 0.081 |
| Platelet | 271.25±63.73 | 263.94±76.37 | 0.563 |
| RDW | 14.84±1.76 | 14.9143±2.02 | 0.844 |
| NLR | 3.21±3.60 | 1.61±2.91 | 0.001 |
| Eos# | 0.17±0.17 | 0.2290±0.21 | 0.162 |
| Eos% | 2.62±1.78 | 2.89±1.77 | 0.423 |
| Baso# | 0.02±0.06 | 0.02±0.04 | 0.860 |
| Baso% | 0.75±0.50 | 0.74±1.93 | 0.423 |
All parameters are expressed as mean±SD values unless otherwise stated. p < 0.05 was accepted as the level of significance. Mann–Whitney test was used to compare the two groups
White Blood cells (WBC), Red Blood Cells (RBC), Haemoglobin (Hgb), Haematocrit (Hct), Red Cell Distribution Width (RDW), Neutrophil Lymphocyte Ratio (NLR), Eosinophils (Eos), Basophils (Baso).
Biochemistry data of the case and control groups.
| Variable | Cases | Controls | p-value |
|---|
| Mean±S.D. | Mean±S.D. |
|---|
| ALT (U/l) | 30.82±19.50 | 29.15±22.70 | .581 |
| AST (U/l) | 19.54±8.50 | 19.53±13.73 | .884 |
| AlkPhos (U/l) | 79.22±32.36 | 86.87±78.55 | .538 |
| Glucose (mmol/l) | 5.08±2.53 | 6.72±3.84 | .024 |
| Ca+ (mmol/l) | 1.47±1.06 | 1.95±0.838 | .045 |
| K+ (mmol/l) | 3.81±1.24 | 4.13±0.90 | .415 |
| Na+ (mmol/l) | 127.33±36.20 | 115.84±50.1 | .139 |
| Cl- (mmol/l) | 94.69±26.97 | 84.00±38.21 | .069 |
All parameters are expressed as mean±SD values unless otherwise stated. P < 0.05 was accepted as the level of significance. Mann–Whitney test was used to compare the two groups.
Alanine transferase (ALT), Aspartate Aminotransferase (AST), Alkaline Phosphatase (AlkPhos), Calcium (Ca+), Potassium (K+), Sodium (Na+), Chloride (Cl-).
When Mann–Whitney U test was performed with gender as factor, in MS patients the RDW (p=0.024) was significantly higher in females, and RBC (p<0.001), Hgb (p<0.001), and Hct (p<0.001), were significantly lower in the females compared to males. There were no significant differences between gender within case group for NLR and vitamin D [Table/Fig-7].
Laboratory data among gender in MS group.
| Variable | Gender | Mean±S.D. | p-value |
|---|
| NLR | Males | 3.607±3.543 | 0.668 |
| Females | 3.125±3.642 |
| Vitamin D | Males | 58.653±31.424 | 0.577 |
| Females | 65.944±44.269 |
| RBC | Males | 5.046±0.357 | <0.001 |
| Females | 4.410±0.478 |
| Hgb | Males | 144.461±13.010 | <0.001 |
| Females | 119.600±15.547 |
| Hct | Males | 42.707±3.954 | <0.001 |
| Females | 36.305±4.237 |
| RDW | Males | 13.861±0.816 | 0.024 |
| Females | 15.080±1.856 |
All parameters are expressed as mean±SD values unless otherwise stated. p < 0.05 was accepted as the level of significance. Mann–Whitney U test was used among the MS group for gender.
Neutrophil to Lymphocyte Ratio (NLR), Red Blood Cells (RBC), Haemoglobin (Hgb), Haematocrit (Hct), Red Cell Distribution Width (RDW).
Discussion
The main findings of our study are as follows: MS group have higher NLR, Cl- and Na+ and, lower Ca+ and glucose as compared to healthy controls. Furthermore, NLR was positively correlated with stress score. This study showed the relationship between NLR and stress score in MS.
MS is a disease of axonal degeneration and demyelination leading to unalterable damage to the CNS. The transfer of autoreactive T cells from the blood to the CNS is the key moment in the pathogenesis of MS, which starts a whole flow of imbalances [19,20]. Previous studies have reported that increased neutrophils in MS patients were related to increased number of Toll-Like Receptor 2 (TLR2), and exhibited phenotypic changes for Formyl Peptide Receptor 1 (FPFR1), Chemokine Receptor Type 4 (CXCR) and Cluster of Differentiation 43 (CD43) expression [19-21]. Previously in MS patients during clinical relapses, clues of neutrophil priming [21], expressing greater levels of medullasin [22] and neutral protease [23] have been demonstrated. An evaluation of the level of inflammatory status may help to determine severity of disease. We have observed in this study that NLR was positively correlated with stress score in MS patients.
In various chronic inflammatory diseases, the NLR, which characterizes the balance between neutrophil and lymphocyte levels, has been proposed as informative in systemic inflammatory conditions [24-28]. Patients of stroke [29] and Parkinson’s disease [30] have elevated NLRs compared to controls. In this study, we found significantly higher NLRs in MS patients than in control group. It appears that the inflammatory response of the CNS in MS may be dependent on the peripheral immune compartment. In Saudi Arabia, the incidence of MS is among the highest in the world. Calculating NLR is simpler and cheaper than measuring other inflammatory cytokines, such as Interleukin-6 (IL-6), Interleukin-1β (IL-1β) and Tumour Necrosis Factor-α (TNF-α) [11,12,31-34].
A major limitation of our study is that the sample size was small. Further investigation with large sample size could overcome this limitation.
Conclusion
Novel biomarkers are desired to suggest the level of inflammation in MS. To the best of our knowledge, this is the first clinical study to demonstrate the increased levels of NLR and stress in MS patients. Hence, NLR could be considered a quicker and cheaper marker with routine CBC analysis for inflammatory marker for MS patients.
Competing interest: "The authors declare that they have no competing interests."
Funding: "The authors declare that funding body did not play role in the design of the study and collection, analysis, and interpretation of data and in writing the manuscript"
* Pearson’s correlation test
*. Correlation is significant at the 0.05 level (two-tailed).
**. Correlation is heighly significant at the 0.001 level (two-tailed).
All parameters are expressed as mean±SD values unless otherwise stated. p < 0.05 was accepted as the level of significance. Mann–Whitney test was used to compare the two groups
White Blood cells (WBC), Red Blood Cells (RBC), Haemoglobin (Hgb), Haematocrit (Hct), Red Cell Distribution Width (RDW), Neutrophil Lymphocyte Ratio (NLR), Eosinophils (Eos), Basophils (Baso).
All parameters are expressed as mean±SD values unless otherwise stated. P < 0.05 was accepted as the level of significance. Mann–Whitney test was used to compare the two groups.
Alanine transferase (ALT), Aspartate Aminotransferase (AST), Alkaline Phosphatase (AlkPhos), Calcium (Ca+), Potassium (K+), Sodium (Na+), Chloride (Cl-).
All parameters are expressed as mean±SD values unless otherwise stated. p < 0.05 was accepted as the level of significance. Mann–Whitney U test was used among the MS group for gender.
Neutrophil to Lymphocyte Ratio (NLR), Red Blood Cells (RBC), Haemoglobin (Hgb), Haematocrit (Hct), Red Cell Distribution Width (RDW).
[1]. Compston A, Coles A, Multiple sclerosisLancet 2008 372(9648):1502-17. [Google Scholar]
[2]. Lauer K, Environmental risk factors in multiple sclerosisExpert Rev Neurother 2010 10(3):421-40. [Google Scholar]
[3]. Tillack K, Naegele M, Haueis C, Schippling S, Wandinger KP, Martin R, Gender differences in circulating levels of neutrophil extracellular traps in serum of multiple sclerosis patientsJ Neuroimmunol 2013 261(1-2):108-19. [Google Scholar]
[4]. Granieri E, Casetta I, Tola MR, Ferrante P, Multiple sclerosis: Infectious hypothesisNeurol Sci 2001 22(2):179-85. [Google Scholar]
[5]. Semiz M, Yildirim O, Canan F, Demir S, Hasbek E, Tuman TC, Elevated neutrophil/lymphocyte ratio in patients with schizophreniaPsychiatr Danub 2014 26(3):220-25. [Google Scholar]
[6]. Fulop T, Tapolyai M, Beauty in simplicity: Abnormal neutrophil to lymphocyte ratio in resistant hypertensionJ Clin Hypertens (Greenwich) 2015 17(7):538-40. [Google Scholar]
[7]. Nunez J, Nunez E, Bodi V, Sanchis J, Minana G, Mainar L, Usefulness of the neutrophil to lymphocyte ratio in predicting long-term mortality in ST segment elevation myocardial infarctionAm J Cardiol 2008 101(6):747-52. [Google Scholar]
[8]. Gunay E, Sarinc Ulasli S, Akar O, Ahsen A, Gunay S, Koyuncu T, Neutrophil-to-lymphocyte ratio in chronic obstructive pulmonary disease: A retrospective studyInflammation 2014 37(2):374-80. [Google Scholar]
[9]. Cheng H, Long F, Jaiswar M, Yang L, Wang C, Zhou Z, Prognostic role of the neutrophil-to-lymphocyte ratio in pancreatic cancer: A meta-analysisSci Rep 2015 5:11026 [Google Scholar]
[10]. Demirci S, Demirci S, Kutluhan S, Koyuncuoglu HR, Yurekli VA, The clinical significance of the neutrophil-to-lymphocyte ratio in multiple sclerosisInt J Neurosci 2016 126(8):700-06. [Google Scholar]
[11]. Martins TB, Rose JW, Jaskowski TD, Wilson AR, Husebye D, Seraj HS, Analysis of proinflammatory and anti-inflammatory cytokine serum concentrations in patients with multiple sclerosis by using a multiplexed immunoassayAm J Clin Pathol 2011 136(5):696-704. [Google Scholar]
[12]. Uysal S, Meric Yilmaz F, Bogdaycioglu N, Mungan Ozturk S, Ak F, Increased serum levels of some inflammatory markers in patients with multiple sclerosisMinerva Med 2014 105(3):229-35. [Google Scholar]
[13]. Beiske AG, Svensson E, Sandanger I, Czujko B, Pedersen ED, Aarseth JH, Depression and anxiety amongst multiple sclerosis patientsEur J Neurol 2008 15(3):239-45. [Google Scholar]
[14]. Zigmond AS, Snaith RP, The hospital anxiety and depression scaleActa Psychiatr Scand 1983 67(6):361-70. [Google Scholar]
[15]. Giordano A, Granella F, Lugaresi A, Martinelli V, Trojano M, Confalonieri P, Anxiety and depression in multiple sclerosis patients around diagnosisJ Neurol Sci 2011 307(1-2):86-91. [Google Scholar]
[16]. da Silva AM, Vilhena E, Lopes A, Santos E, Goncalves MA, Pinto C, Depression and anxiety in a Portuguese MS population: Associations with physical disability and severity of diseaseJ Neurol Sci 2011 306(1-2):66-70. [Google Scholar]
[17]. Espinola-Nadurille M, Colin-Piana R, Ramirez-Bermudez J, Lopez-Gomez M, Flores J, Arrambide G, Mental disorders in mexican patients with multiple sclerosisJ Neuropsych Clin N 2010 22(1):63-69. [Google Scholar]
[18]. Bayani AA, Reliability and preliminary evidence of validity of a farsi version of the depression anxiety stress scalesPercept Motor Skill 2010 111(1):107-14. [Google Scholar]
[19]. Meinl E, Krumbholz M, Hohlfeld R, B lineage cells in the inflammatory central nervous system environment: migration, maintenance, local antibody production, and therapeutic modulationAnn Neurol 2006 59(6):880-92. [Google Scholar]
[20]. Naegele M, Tillack K, Reinhardt S, Schippling S, Martin R, Sospedra M, Neutrophils in multiple sclerosis are characterized by a primed phenotypeJ Neuroimmunol 2012 242(1-2):60-71. [Google Scholar]
[21]. Ziaber J, Pasnik J, Baj Z, Pokoca L, Chmielewski H, Tchorzewski H, The immunoregulatory abilities of polymorphonuclear neutrophils in the course of multiple sclerosisMediators Inflamm 1998 7(5):335-38. [Google Scholar]
[22]. Aoki Y, Miyatake T, Shimizu N, Yoshida M, Medullasin activity in granulocytes of patients with multiple sclerosisAnn Neurol 1984 15(3):245-49. [Google Scholar]
[23]. Guarnieri B, Lolli F, Amaducci L, Polymorphonuclear neutral protease activity in multiple sclerosis and other diseasesAnn Neurol 1985 18(5):620-22. [Google Scholar]
[24]. Dirican N, Anar C, Kaya S, Bircan HA, Colar HH, Cakir M, The clinical significance of hematologic parameters in patients with sarcoidosisClin Respir J 2016 10(1):32-39. [Google Scholar]
[25]. Fowler AJ, Agha RA, Neutrophil/lymphocyte ratio is related to the severity of coronary artery disease and clinical outcome in patients undergoing angiography—the growing versatility of NLRAtherosclerosis 2013 228(1):44-45. [Google Scholar]
[26]. Kuyumcu ME, Yesil Y, Ozturk ZA, Kizilarslanoglu C, Etgul S, Halil M, The evaluation of neutrophil-lymphocyte ratio in Alzheimer’s diseaseDement Geriatr Cogn Disord 2012 34(2):69-74. [Google Scholar]
[27]. Okyay GU, Inal S, Onec K, Er RE, Pasaoglu O, Pasaoglu H, Neutrophil to lymphocyte ratio in evaluation of inflammation in patients with chronic kidney diseaseRen Fail 2013 35(1):29-36. [Google Scholar]
[28]. Sen BB, Rifaioglu EN, Ekiz O, Inan MU, Sen T, Sen N, Neutrophil to lymphocyte ratio as a measure of systemic inflammation in psoriasisCutan Ocul Toxicol 2014 33(3):223-27. [Google Scholar]
[29]. Gokhan S, Ozhasenekler A, Mansur Durgun H, Akil E, Ustundag M, Orak M, Neutrophil lymphocyte ratios in stroke subtypes and transient ischemic attackEur Rev Med Pharmacol Sci 2013 17(5):653-57. [Google Scholar]
[30]. Akil E, Bulut A, Kaplan I, Ozdemir HH, Arslan D, Aluclu MU, The increase of carcinoembryonic antigen (CEA), high-sensitivity C-reactive protein, and neutrophil/lymphocyte ratio in Parkinson’s diseaseNeurol Sci 2015 36(3):423-28. [Google Scholar]
[31]. Arnason BG, Dayal A, Qu ZX, Jensen MA, Genc K, Reder AT, Mechanisms of action of interferon-beta in multiple sclerosisSpringer Semin Immunopathol 1996 18(1):125-48. [Google Scholar]
[32]. Ishizu T, Osoegawa M, Mei FJ, Kikuchi H, Tanaka M, Takakura Y, Intrathecal activation of the IL-17/IL-8 axis in opticospinal multiple sclerosisBrain 2005 128(Pt 5):988-1002. [Google Scholar]
[33]. Matejcikova Z, Mares J, Prikrylova Vranova H, Klosova J, Sladkova V, Dolakova J, Cerebrospinal fluid inflammatory markers in patients with multiple sclerosis: A pilot studyJ Neural Transm (Vienna) 2015 122(2):273-77. [Google Scholar]
[34]. McMillan SA, McDonnell GV, Douglas JP, Droogan AG, Hawkins SA, Elevated serum and CSF levels of soluble CD30 during clinical remission in multiple sclerosisNeurology 1998 51(4):1156-60. [Google Scholar]