Hypertension is a key modifiable risk factor for cardiovascular mortality and morbidity and it is considered to be silent diseases, whose symptoms are not noticeable until and unless the disease is in an advanced phase. Globally, one billion people are suffering from hypertension [1]. In India, about 33% urban and 25% rural populations are hypertensive [2]. The increase in the prevalence of hypertension is dependent on numerous interlinked factors such as urbanization with associated changes in lifestyle and food habits, ageing and social stress [2].
Hypertension management can be achieved by drug therapy or change in lifestyle or both. Lifestyle changes have been proposed to manage hypertension, these include a reduction in weights, limiting salt intake, increases the physical activity, and restriction of alcohol intake [3].
As for the JNC-8, guideline CCBs are the primary antihypertensive drugs. There are two types of CCBs present; depending on the chemical structure they are classified into dihydropyridine and non-dihydropyridine groups. There are different type of calcium channels present in our body, such as L, N, T, P/Q, and R-type [3]. Pharmacokinetics and pharmacodynamics property vary between different classes of CCBs [4].
Amlodipine is third generation CCB with an excellent pharmacological profile. The major drawback of amlodipine is, it induces pedal oedema [3]. Chronic therapy of amlodipine enhances the release of more catecholamines from sympathetic nerve terminals [5], few clinical studies showed that amlodipine enhances the release of more endothelial nitric oxides [6], and decreases the Atrial Natriuretic Peptide (ANP) [7]. Cilnidipine is a fourth generation L/N type of CCB [8], which blocks the N-type of calcium channels at the sympathetic nerve endings and decreases the release of catecholamines and by blocking L-type calcium channels relaxes arteriolar smooth muscles, which decreases the peripheral vascular resistance [9,10]. In the kidney, cilnidipine reduces the proteinuria by relaxing both afferent and efferent arterioles [11,12]. Although, there are many studies available on amlodipine and cilnidipine therapy, there are no specific studies on the comparison of electrocardiographic changes, echocardiographic, biochemical parameters between the groups. Hence, we carried this study to compare the clinical, and biochemical parameters, with amlodipine and cilnidipine in essential hypertensive patients.
Materials and Methods
Ethics
The present study was held in the Department of Cardiology, Kasturba Medical College Hospital, Manipal University, Manipal, Karnataka, India. The study duration was three years (Jan 2014 to Dec 2016). A total of one hundred forty hypertensive patients of either gender attending the outpatient department of cardiology and medicine recruited for this study. The patient's information sheet was given to all patients and explained about the present study; written informed consent was obtained from all the study participants before study commences. The study protocol was confirmed, and approval of the Institutional Ethics Committee (Approval no IEC 681/2013). The present study conducted at Kasturba Hospital, Manipal, Karnataka, India.
Study Design
The present study was a cross-sectional study, between two antihypertensive drug therapy groups at Kasturba Hospital, Manipal, Karnataka, India. A total of 140 mild to moderate hypertensive patients (HTN classified according to JNC-8 HTN guideline), 70 were in amlodipine group (Group-A), and other 70 patients were in cilnidipine group (Group-B). Amlodipine group (Group-A) receiving Tab. Amlodipine 5 mg/day and cilnidipine group (Group-B) receiving Tab. 10 mg/day, since more than six months. Patients enrolled into the study with due consideration of eligibility criteria. Demographic, clinical and biochemical parameters were noted and compared.
Sample Size Calculation
Sample size calculations were made by based on the following formula
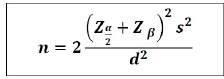
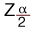 = 1.96 for 95% confidence level.
= 1.96 for 95% confidence level.
Zβ= 1.28 for 90% power.
S2 = Pooled standard deviation observation of two samples. (3.5 x 3.5=12.24)
d2 = Clinically significant difference. (2 x 2 = 4)
Considering an error of 5% with a 95% confidence level and 90% of power, then the minimum required sample size will be 65 on each arm. In the present study, we have taken 70 patients in each arm.
Inclusion and Exclusion Criteria
Essential hypertensive patients of both genders, patients currently receiving amlodipine 5 mg/day since more than six months for amlodipine group (Group-A), and cilnidipine 10 mg/day since six months for cilnidipine group (Group-B) as an antihypertensive therapy. The age limit was 18 to 70 years. Patients with any endocrine abnormalities, severe infections, kidney diseases, liver disease, pregnant women, lactating women and patients on other class antihypertensive medications except amlodipine and cilnidipine were excluded from the study.
Study procedure
A total of 140 patients were recruited in this study, who met the inclusion criteria. The consultant cardiologist examined the patients. The blood pressure was measured using a standard mercury sphygmomanometer, and the pulse rate was noted (mean of three readings were noted in 10 minutes interval of time). After initial screening, demographic parameters, family history, clinical findings, electrocardiogram data, echocardiography findings were noted. The biochemical parameters are serum creatinine, albumin, globulin, total protein, sodium, osmolality, fractional excretion of sodium and 24 hours. VMA readings were noted and compared between two study groups. Hence, it was a cross-sectional study; we did not do the follow-up the patients, and during the study, we did not observe any adverse effect.
Statistical Analysis
Using SPSS software (version 15.0). Values are expressed as mean±SD, Inter-Quartile Range (IQR) and percentage. The difference of the demographic parameter between the two groups was compared using an independent t-test. The Mann-Whitney test was used to compare the skewed variables. A p-value <0.05 considered statistically significant.
Results
The present study was a cross-sectional study. Hence all recruited patients have completed the study. Patient’s age for both the groups ranged between 45 to 70 years, with the mean age being 57.96±8.6 years and 57.79±10.07 years in amlodipine and cilnidipine groups respectively. In amlodipine group, 43 males and 27 female patients and cilnidipine group 37 males and 33 female patients were enrolled. Other demographic parameters are like smoking status, family history of Coronary Artery Diseases (CAD) are well matched, and there were no significant changes between the two study groups, (p > 0.05), which are noted in [Table/Fig-1].
Demographic parameters of amlodipine and cilnidipine groups
| Sl.No | Varibles | Groups | p-value* |
|---|
| Amlodipine | Cilnidipine |
|---|
| 1 | Age(years) | Mean ± SD | 57.96 ±8.65 | 57.79±10.07 | 0.914 |
| 2 | Gender(M/F) | Range | 45 -70 | 46 -70 |
| Male | 43(61.43%) | 37(52.86%) | 0.306 |
| Female | 27(38.57%) | 33(47.14%) |
| 3 | Height (cm) | Mean ± SD | 159.21±9.50 | 157.27±10.60 | 0.259 |
| Range | 131-176 | 142-172 |
| 4 | Diabetic | DM | 29(41.43%) | 32(45.71%) | 0.128 |
| Non-DM | 41(58.57%) | 38(54.29%) |
| 5 | Dyslipidemia | Present | 26(37.14%) | 41(58.57%) | 0.604 |
| Absent | 44(62.86%) | 29(41.43%) |
| 6 | Smoking status | Smokers | 24(34.29%) | 16(22.86%) | 0.134 |
| Non-smokers | 46(65.71%) | 54(77.14%) |
| 7 | Family history of CAD | CAD | 23(32.85%) | 19(27.14%) | 0.461 |
| Non-CAD | 47(67.14%) | 51(72.86%) |
Categorical variables were compared using Chi-square test and continous variables were compared by Independent t-test. p<0.05 considered as significant.
Intergroup Analysis
Amlodipine treated hypertensive group: In this group 29 (41.43%) diabetic and 41 (58.57%) non diabetic patients were enrolled, and 26 (37.14%) were dyslipidemic and 44 (62.86%) patients were non-dyslipidemic, both diabetic and dyslipidemic patients were on medications. The clinical and biochemical parameters are compared using an independent t-test, there was no significant difference seen (p>0.05).
The anti-diabetic drugs used were Human insulin mixtard six patients (21%), sulfonylureas ten patient (34%), meglitinides 11 patients (38%), thiazolidinedione two patients (9%) were using since more than one year [Table/Fig-2].
Pie-diagram showing anti diabetic and anti dyslipidemic drugs used in amlodipine and cilnidipine treated hypertensive group.
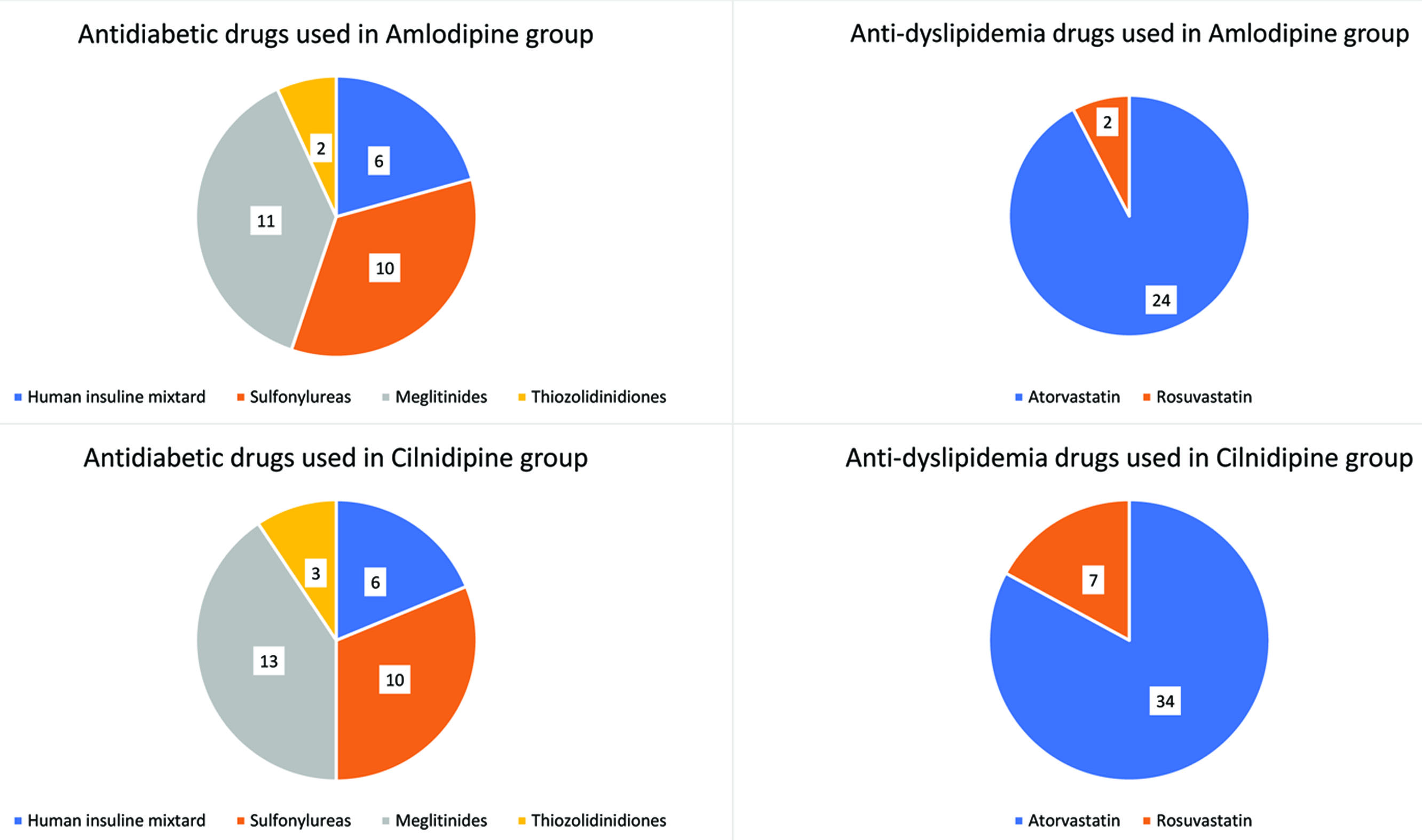
The anti-dyslipidemic drugs used are atorvastatin 24 patients (92%) and rosuvastatin only two patients were using (8%) since more than one year [Table/Fig-2].
Cilnidipine treated hypertensive group: In this group 32 (45.71%) diabetic and 38 (54.29%) non diabetic patients were enrolled, and 41 (58.57%) were dyslipidemic and 29 (41.43%) patients were non-dyslipidemic, both diabetic and dyslipidemic patients were on medications. The clinical and biochemical parameters were compared using an independent t-test, there was no significant difference seen (p>0.05).
The anti-diabetic drugs used were Human insulin mixtard six patients (19%), sulfonylureas by ten patient (31%), meglitinides by 13 patients (41%), thiazolidinedione three patients (9%), were using since more than one year [Table/Fig-2].
The anti-dyslipidemic drugs used are atorvastatin was using by 34 patients (83%) and rosuvastatin were using by seven patients (17%) since more than one year [Table/Fig-2].
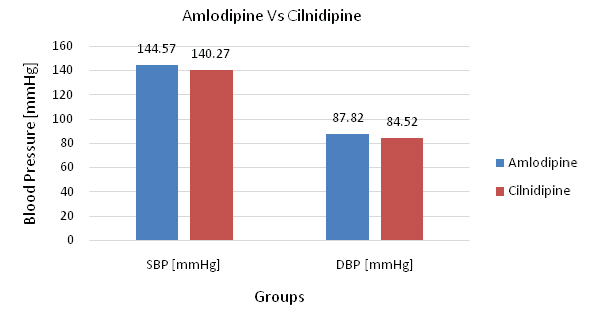
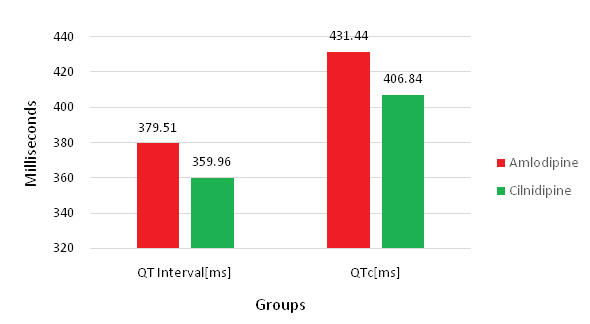
Systolic and diastolic Blood Pressure (BP) showed a significant difference between amlodipine and cilnidipine treated hypertensive groups (p<0.001) [Table/Fig-3]. Where cilnidipine treated hypertensive group showed a 4.3 mmHg SBP and 3.3 mmHg DBP reduction than the amlodipine treated hypertensive group [Table/Fig-4]. There was no significant difference in pulse rate between the two groups. Average QT interval between two groups showed a statistically significant difference, but within the normal range. Cilnidipine treated hypertensive group showed 19.55 milliseconds reduction in QT interval time than the amlodipine group [Table/Fig-5]. Ejection Fraction (EF) and diameter of inferior vena cava during expiration did not show any significant change between two hypertensive groups. Clinical and echocardiographic parameters are detailed in [Table/Fig-3]. Biochemical parameters are serum creatinine (mg/dL), serum albumin (g/dL), serum globulin (g/dL), total protein (g/dL) serum Na+ (mmol/L), fractional excretion of Na+(%), serum osmolality (mosml/kg), VMA (mg/24 hour) readings were noted and compared between two study groups, there was no significant difference seen, which is explained in [Table/Fig-6].
Comparison of clinical and echocardiography parameters between amlodipine and cilnidipine groups.
| Sl.No | Variables | Amlodipine | Cilnidipine | p-value* |
|---|
| (mean ± SD) | (mean ± SD) |
|---|
| 1 | Systolic BP (mmHg) | 144.57±4.58 | 140.27±4.06 | < 0.001 |
| 2 | Diastolic BP (mmHg) | 87.82±3.78 | 84.52±4.4 | < 0.001 |
| 3 | Pulse rate (breath/minute) | 78.30±10.80 | 76.70±9.73 | 0.359 |
| 4 | QT interval (ms) | 379.51±29.82 | 359.96±25.24 | < 0.001 |
| 5 | QTc (ms) | 431.44±28.974 | 406.84±27.701 | < 0.001 |
| 6 | Left Ventricle (LV) Ejection Fraction (%) | 64.66±3.097 | 65.33±4.30 | 0.291 |
| 7 | Inferior Vena Cava (IVC) (mm) | 14.93±1.48 | 15.27±1.19 | 0.134 |
Variables are compared by independent t-test. p<0.05
Bar-diagram showing comparison of systolic and diastolic blood pressure (SBP and DBP) between amlodipine and cilnidipine group, were cilnidipine group showed significant reduction in SBP/DBP than amlodipine group. (p<0.001).
Bar-diagram showing comparison of QT/QTc between amlodipine and cilnidipine group, were cilnidipine group showed statistically significant shortened in QT/QTc than amlodipine group.(p<0.001).
Comparison of biochemical variables between amlodipine and cilnidipine group.(Blood and urine sample).
| Sl. No | Blood and urine sample variables | Amlodipine (n=70) | Cilnidipine (n=70) | p-value* |
|---|
| (mean ± SD) | (mean ± SD) |
|---|
| 1 | Serum Creatinine (mg/dL) | 0.95±0.19 | 1±0.17 | 0.112 |
| 2 | Serum Albumin (gm/dL) | 4.32±0.38 | 4.37±0.19 | 0.262 |
| 3 | Serum Globulin (gm/dL) | 3.16±0.56 | 3.17±0.40 | 0.973 |
| 4 | Total Protein (gm/dL) | 7.48±0.55 | 7.49 ±0.38 | 0.493 |
| 5 | Serum Na+ (mmol/L) | 138.82±3.35 | 139.57±2.79 | 0.156 |
| 6 | Fractional Excretion of Na+(%) | 0.22±0.31 | 0.26±0.44 | 0.559 |
| 7 | Serum Osmolality (mosml/kg) | 288.71±10.69 | 278.14 ± 11.87 | 0.708 |
| 8 | VMA (mg/24 hours.) | 5.15±1.87 | 5.01±1.58 | 0.645 |
Continuous variables are compared by independent t-test. p<0.05
Discussion
Dihydropyridine group of CCBs is most commonly used antihypertensive agents to treat essential hypertension [8]. According to JNC-8 recommended CCBs are the first line antihypertensive drugs [13]. Antihypertensive effect CCBs depends on their mechanism of action. Amlodipine is the third generation CCB which acts on L-type of calcium channels at vascular smooth muscles and relaxes the vascular smooth muscles and by this action reduces the peripheral vascular resistance; this leads to a reduction in blood pressure [3]. Cilnidipine is a fourth generation CCB which acts on both L-type calcium channels in vascular smooth muscle and relaxes and N- type calcium channels at neuronal terminals reduces the release of catecholamines, by this unique mechanism it reduces the blood pressure [8]. Cilnidipine have a beneficiary effect on kidney, neurones and cardiovascular system, these effects have been demonstrated in clinical practice or animal experiments [8,9]. In the present study, we found that the cilnidipine treated group showed better blood pressure control than the amlodipine group and there was no significant change in pulse rate. Previously many studies have been reported on safety and efficacy of amlodipine and cilnidipine associated with minor adverse effects such as a headache, dizziness, cough and gastrointestinal symptoms [10,12]. Ejection fraction was compared between two study groups, but there were no significant changes seen. Amlodipine and cilnidipine did not show any significant difference in plasma proteins. The chronic therapy of amlodipine enhances release of more catecholamines from the nerve terminals [5], but in the present study, Vanillyl mandelic acid did not show a significant difference between the two study groups. By this study result, we can say that both amlodipine and cilnidipine are similar actions on sympathetic nervous system. A meta-analysis conducted by Guo-Liang X et al., on efficacy and safety of cilnidipine in Chinese patients, in essential hypertension and concluded that cilnidipine showed equally effective as amlodipine in blood pressure control, and also cilnidipine showed the better safety profile [14]. Hoshide S et al., conducted a study to compare the 24 hours ambulatory BP and Pulse Rate (PR) they concluded that cilnidipine treated group showed a significant reduction in ambulatory BP and PR than the amlodipine group [15]. In the present study, we noted the average QT interval from the 12 lead ECG and QTc was calculated from Bazett’s formula between two study groups. We found that in cilnidipine group showed statistically significant shortened QT/QTc interval than amlodipine treated group. Takahara A et al., reported an animal study that the QT interval and monophasic action potential duration were shortened in cilnidipine treated group but it was not noticed in an amlodipine treated group [4]. Cilnidipine attenuated the sympathetic over-activity and shortens the prolonged QT/QTc and reduces the blood pressure and PR [16]. We noticed the prominent P wave in cilnidipine treated group than the amlodipine group. The major drawback of amlodipine is the incidence of pedal oedema [3], in such case cilnidipine is more benefit drug with a lesser incidence of pedal oedema and shows the better hypertension control [1]. Cilnidipine is more beneficial than amlodipine as an additional medication for hypertensive patients with Chronic Kidney Diseases (CKD) [17]. Amlodipine and cilnidipine are the potential antihypertensive agents with the good pharmacological profile.
Limitation
The present study was a cross-sectional study. Hence, there was no follow up of the patients. A single visit took into consideration in both the groups; this might have led to some bias. We enrolled outpatients, so we did not concentrate on their diet, water and salt intake; this may alter the results. Since we enrolled diabetic as well as dyslipidemia patients, the drug used for treatment may vary the clinical and biochemical parameters, but in the present study there was no significant difference seen between diabetic and non-diabetic, dyslipidemic and non-dyslipidemic in amlodipine and cilnidipine treated hypertensive patients.
Conclusion
The amlodipine and cilnidipine both are equally effective antihypertensive drugs. Cilnidipine treated group showed more reduction in blood pressure than the amlodipine treated group and there was no significant change in heart rate between the two groups. Cilnidipine group showed shortened QT/QTc interval than the amlodipine group.
Abbreviations
HTN : Hypertension
BP : Blood Pressure
PR : Pulse Rate
VMA : Vanillyl Mandelic Acid
CKD : Chronic Kidney Diseases
CCB : Calcium Channel Blocker
*Categorical variables were compared using Chi-square test and continous variables were compared by Independent t-test. p<0.05 considered as significant.