Shafer defined dental caries as, an irreversible microbial disease of the calcified tissues of the teeth, characterized by demineralisation of the inorganic portion and destruction of the organic substance of the tooth, which often leads to cavitation. It is a complex and dynamic process where a multitude of factors influence and initiate the progression of disease [1]. Although research has aided to further our knowledge of dental caries and reduce its prevalence, according to recent reports, dental caries still remains a major dental disease [2].
Demineralisation and remineralisation are balanced processes that normally occur in the oral cavity. Sometimes, diet variations, oral hygiene or microbial activity can lead to the predominance of demineralisation. Remineralisation is facilitated by the buffering action of saliva, permitting calcium and phosphate ions to precipitate onto the tooth and form new mineral [3,4]. Therefore, modulation of the demineralisation-remineralisation balance is the key to prevention of dental caries.
Until recently, the conventional treatment concept for all carious teeth involved caries excavation and replacement with a restorative material [5]. However, with decades of research, evolved the “minimally invasive” approach which incorporates detecting and treating these areas sooner, emphasizing on prevention, rather than the traditional surgical model [4].
Fluoride has been recognized as the main propriety for the decline in caries due to its cariostatic potential [6]. Despite its profound effect in halting caries progression, it has been met with certain limitations. Fluoride does not aid in eliminating caries totally. Moreover, increased fluoride concentration can produce detrimental effects on the tooth [7].
Furthermore, the availability of calcium and phosphate ions can be a limiting factor for fluoride retention and for net remineralisation to occur [6]. This requirement has recoursed research to develop preventive agents functioning as an adjunct/independent to fluoride [8,9].
The anticariogenic potential and remineralising properties of CPP-ACP derived from milk protein casein have been exhibited in vitro and in situ studies [10]. The CPP-ACP acts as reservoir of bio-available calcium and phosphate, thus facilitating remineralisation [11]. GC Tooth Mousse containing 0.09% fluoride is available as CPP–ACPF paste (GC Tooth Mousse Plus; GC Corporation, Tokyo, Japan). CPP–ACPF has been reported to have a greater potential for remineralisation than CPP–ACP [12-16].
NovaMin (SHY-NM, Group Pharmaceuticals Ltd., India) a BAG has the ability to act as a biomimetic mineralizer [3]. There is a multitude of research to support NovaMin as a successful desensitizing agent [17-21]. However, only a few studies are available to validate the remineralisation action of NovaMin on enamel [22-25].
Remin Pro (VOCO, Gmbh, Germany) is yet another remineralising paste which in contrast to CPP-ACP products contains calcium and phosphate in the hydroxyapatite form. In addition, fluoride and xylitol have also been included in this product [26]. Heshmat H et al., and Kamath U et al., reported significant increase in microhardness of enamel after bleaching [26,27].
Recently, scientists from the University of Leeds developed a patented technology for regeneration of enamel: The Curolox technology. P11-4 is a rationally-designed peptide, the monomers of which undergo well characterised self-assembly into a biocompatible fibrillar scaffold in response to specific environmental triggers that mimics the enamel matrix. Around this matrix, enamel crystals are formed from calcium phosphate from the saliva [28-30]. Curodont Protect (Credentis, Switzerland), is a combination of the active ingredients fluoride, calcium phosphate and protein molecules [30].
To date, no study has compared the remineralisation potential of CPP-ACPF, BAG, fluoride enhanced HA gel and self-assembling peptide P11-4. Therefore, the present in vitro study was designed to evaluate the remineralising capacity of the above agents on artificial enamel lesions, through Surface Microhardness (SMH) analysis and SEM examination.
Materials and Methods
This in-vitro prospective study was conducted over a period of one month in the Department of Conservative dentistry and Endodontics, Goa Dental College and Hospital, Goa, India. A total of 60 human maxillary and mandibular premolars which were extracted for orthodontic purposes were selected for the study. Teeth with any visible or detectable caries, restorations, hypoplastic lesions, stains, cracks and white spot lesions were excluded from the study.
After removal of debris, calculus and soft tissue from the tooth surface, the teeth were stored in 10% formalin solution until further use. Teeth were then sectioned 1 mm below the cementoenamel junction with a slow speed diamond disc. The roots were discarded and the crowns were used for the study. The specimens were stored in antifungal solution containing 0.1% thymol solution until the experimental procedure was initiated.
Custom made plastic cylindrical moulds were prepared and self-cured acrylic resin was poured in them. Each tooth crown was embedded in the resin with the buccal surface facing upward and exposed and parallel to the horizontal plane. The buccal surface was flattened and polished using 400, 800, 1000, 1200 grit abrasive paper sequentially.
A 5 mm × 5 mm window of exposed enamel was created in the middle of the sample surface by using adhesive tape and the sample was rendered resistant to acid attack by applying a uniform coat of the nail varnish around it. Once the samples were adequately dried, the adhesive tape was removed from the tooth surface using an explorer, exhibiting a rectangular area on the enamel surface.
Baseline Surface Microhardness (B-SMH) Measurement
B-SMH was checked with Vicker’s Microhardness Testing machine (VMT) for all the tooth samples in the area of the working window. The indentations were made with VMT at the rate of 100 g load for 10 seconds. The average microhardness of the specimen was determined from three indentations to avoid any discrepancy.
Preparation of Demineralising and Remineralising Solutions [
2]
The buffered de-/re-mineralizing solutions were prepared using analytical grade chemicals and deionized water. The demineralising solution contained 2.2 mM calcium chloride, 2.2 mM potassium phosphate, and 0.05 M acetic acid; the pH was adjusted with 1 M sodium hydroxide to 4.4. The remineralising solution contained 1.5 mM calcium chloride, 0.9 mM sodium phosphate, and 0.15 M potassium chloride, with a pH of 7.0.
Lesion Formation
Each of the samples was individually immersed in the demineralising solution (20 ml) for 96 hours to produce artificial carious lesions in the enamel. After 96 hours of initial Demineralisation, Surface Microhardness values (D-SMH) were checked with VMT, as done for B-SMH.
Division of Groups
A total of 60 samples were randomly divided into five groups of 12 samples each.
Group A: Sound enamel, no treatment (Control);
Group B: CPP-ACPF (Tooth Mousse Plus);
Group C: Bioactive Glass (SHY- NM);
Group D: Fluoride enhanced hydroxyapatite gel (ReminPro);
Group E: Self-Assembling Peptide P11-4 (Curodont Protect).
The pH Cycling Model
A pH cycling model was adopted to simulate the changes occurring in the oral cavity. The remineralising pastes were applied with applicator tips and left on for two minutes, following which the samples were thoroughly washed with deionized water. The samples were then individually immersed in 20 ml of demineralising solution (pH 4.4) for a period of three hours and were then washed with deionized water. This was followed up with treatment of the samples again with the respective remineralising agents for two minutes which was then washed off with deionized water. All the enamel samples were then individually immersed in 20 ml of remineralising solution (pH 7) for a period of 17 hours. The pH cycling was carried out for a period of 30 days. The remineralising and demineralising solutions were replaced every 48 hours and five days respectively. After the culmination of the pH cycling process, all the enamel samples were assessed for SMH using Vickers hardness tester.
SEM Examination
For the SEM examination, three sample specimens in each group were randomly selected and evaluated for surface changes. The scanning electron microscope was used to determine and compare the morphological variations between the different treated samples. For comparison, the surfaces of the sound and demineralised enamel were also examined. Images were obtained at x500 magnification.
Statistical Analysis
The data obtained was subjected to statistical analysis using the SPSS software. The results were analysed by ANOVA. Multiple comparisons between groups were performed by paired t-test and post-hoc Tukey test. For the entire evaluation, p<0.05 was considered to be statistically significant.
Results
Comparison between the B-SMH and D-SMH values is displayed in [Table/Fig-1]. Statistically significant difference was noted, suggesting a decrease in SMH following the simulated demineralisation cycle.
Comparison between baseline (B-SMH) and post demineralisation surface microhardness (D-SMH) values of test groups.
| Paired Samples Statistics |
|---|
| Mean | n | Std. Deviation | Std. Error Mean |
|---|
| Baseline (B-SMH) | 327.88 | 48 | 117.632 | 16.979 |
| Post Demineralisation (D-SMH) | 211.33 | 48 | 115.416 | 16.659 |
| Paired Samples Test |
| Mean difference | Std. Deviation | t | df | Sig. |
| Baseline (B-SMH) - After Demineralisation (D-SMH) | 116.542t | 149.364 | 5.406 | 47 | <0.001 |
One-way ANOVA revealed significant differences between the tested groups [Table/Fig-2]. Multiple comparison between the experimental groups and control is presented in [Table/Fig-3]. The highest post remineralisation SMH values were recorded in the Group E (self assembling peptide P11-4) differing significantly from all the other tested materials except for Group B (CPP-ACPF). There were no statistically significant differences between CPP-ACPF and Group C (BAG). The least amount of surface remineralisation was exhibited by Group D (fluoride enhanced HA gel) differing statistically from self assembling peptide P11-4 and CPP-ACPF group.
Comparison between B-SMH, D-SMH and R-SMH of all the groups.
| Descriptive Statistics |
|---|
| n | Mean | Std. Deviation | Std. Error | 95% Confidence Interval for Mean | Min | Max |
|---|
| Lower Bound | Upper Bound |
|---|
| B-SMH | 60 | 317.80 | 107.857 | 13.924 | 289.94 | 345.66 | 199 | 798 |
| D-SMH | 48 | 211.33 | 115.416 | 16.659 | 177.82 | 244.85 | 102 | 585 |
| GRP B | 12 | 267.75 | 36.940 | 10.664 | 244.28 | 291.22 | 196 | 326 |
| GRP C | 12 | 244.83 | 27.336 | 7.891 | 227.47 | 262.20 | 199 | 289 |
| GRP D | 12 | 229.17 | 23.567 | 6.803 | 214.19 | 244.14 | 199 | 275 |
| GRP E | 12 | 283.67 | 18.406 | 5.313 | 271.97 | 295.36 | 236 | 302 |
| Total | 156 | 266.13 | 103.966 | 8.324 | 249.69 | 282.58 | 102 | 798 |
| ANOVA |
| Sum of Squares | df | Mean Square | F | Sig. |
| Between Groups | 56767.567 | 4 | 14191.892 | 13.370 | <0.001 |
| Within Groups | 58383.167 | 55 | 1061.512 | | |
Comparison of remineralisation values between control and test groups.
| Groups | Mean Difference | Sig |
|---|
| Control | CPP-ACPF | 49.667* | .004 |
| BAG | 72.583* | <0.001 |
| FEHG | 88.250* | <0.001 |
| P11-4 | 33.750 | .097 |
| CPP-ACPF | Control | -49.667* | .004 |
| BAG | 22.917 | .429 |
| FEHG | 38.583* | .041 |
| P11-4 | -15.917 | .753 |
| BAG | Control | -72.583* | <0.001 |
| CPP-ACPF | -22.917 | .429 |
| FEHG | 15.667 | .764 |
| P11-4 | -38.833* | .039 |
| FEHG | Control | -88.250* | <0.001 |
| CPP-ACPF | -38.583* | .041 |
| BAG | -15.667 | .764 |
| P11-4 | -54.500* | .001 |
| P11-4 | Control | -33.750 | .097 |
| CPP-ACPF | 15.917 | .753 |
| BAG | 38.833* | .039 |
| FEHG | 54.500* | .001 |
Post-Hoc Tukey Test
*. The mean difference is significant at the 0.05 level.
CPP-ACPF, Casein phosphopeptide amorphous calcium phosphate fluoride; BAG, Bioactive Glass; FEHG, Fluoride enhanced hydroxyapatite gel; P11-4, Self-Assembling Peptide P11-4.
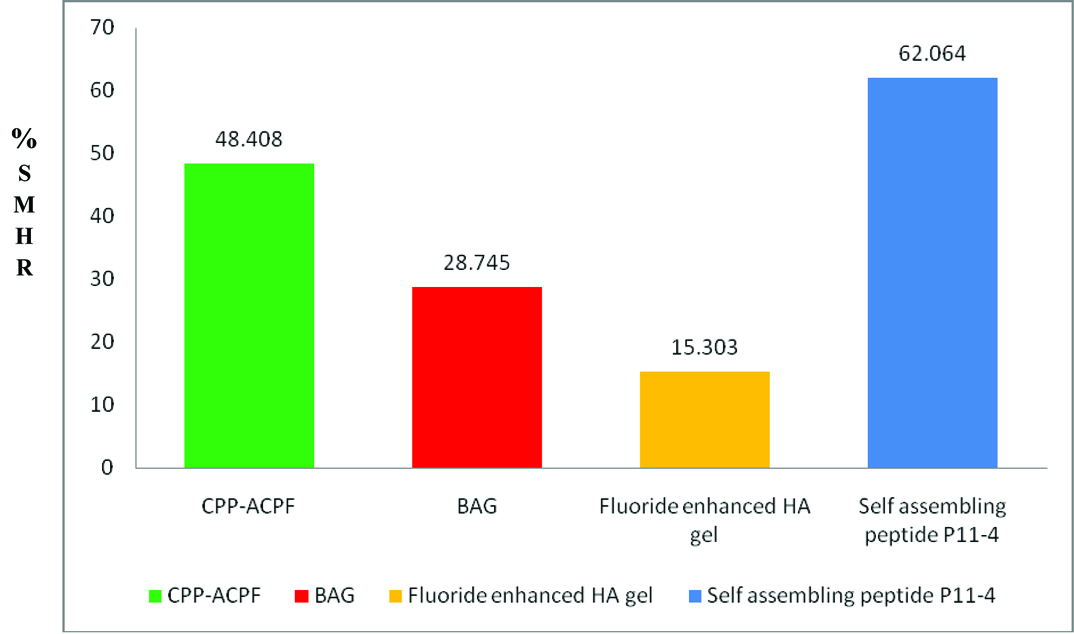
The percentage of SMH recovery was calculated for all the experimental groups. The highest recovery rate of 62.06% was recorded by self assembling peptide P11-4 group, followed by 48.41% in CPP-ACPF group, 28.75% in BAG group and 15.30% in the fluoride enhanced HA gel [Table/Fig-4].
Comparison of percentage of surface microhardness recovery (%SMHR) between the test groups. 
Discussion
Scientific literature proposes that clinical management of tooth demineralisation should emphasize on early detection and prevention, before a restorative approach is applied. Decades of research has lead to the advancement of technologies that can promote enamel remineralisation or down scale demineralisation thereby reinforcing and aiding oral health [31].
Considerable efforts have been made to limit the progression of carious lesions, and while clinical studies remain the gold standard, standardized in vitro models are the most conventional techniques in cariology research and can serve as a valuable tool for assessing anti caries efficacy of remineralising agents [32].
The pH-cycling protocol entails exposure of dental substrates (enamel or dentin) to a series of demineralisation and remineralisation. These studies are designed to mimic the dynamics of mineral loss and gain involved in caries formation [32-34]. The pH cycling protocol adopted for this study was based on the model described by Featherstone JDB et al., [35]. This pH cycling model has been utilised successfully to review the anti caries potential of dentifrice formulations since it simulates in-vivo high caries risk situations and also measures the net result of the inhibition of demineralisation and the enhancement of remineralisation [36]. In the protocol adopted, the dynamic cycles of demineralisation and remineralisation was simulated by sequentially immersing enamel specimens in acidic (demineralising) and supersaturated (remineralising) solutions. Dentifrice use was simulated by topical application of the agents during the de- and remineralisation stages. The demineralising solution uses an acid buffer of pH 4.4, while the remineralisation solution contains calcium and phosphate at a known degree of saturation at pH 7.0. This solution approximates the mineral ion composition and supersaturation of saliva. In this study, the composition of the demineralising and remineralising solutions was similar to the one employed by Buzalaf M et al., [33].
Numerous techniques have been employed for the assessment of enamel remineralisation. This can be achieved either quantitatively by mineral content and hardness profiles or qualitatively by Polarized Light Microscopy (PLM) and SEM [33].
SMH evaluation is a simple, quick and easy to measure non destructive method, reflecting mineral changes that have occurred due to the therapeutic procedures. The method also permits repeated measurements of the same specimen over a given period of time thereby reducing the experimental variation [32]. Taking into account the significance of the surface layer in caries progression, assessing the alterations occurring in this region is relevant, thus, SMH measurement is a suitable technique for studying de-remineralisation process and was therefore employed in this study [2,11].
In the present study, the enamel samples were first tested to obtain the baseline Vickers SMH values. The mean baseline value recorded was 317.80. Following lesion formation, the samples were tested again and the mean value decreased to 211.33 after 96 hours of demineralisation [Table/Fig-2]. The difference in both the values was statistically significant (p<0.05) [Table/Fig-1]. This reduction in SMH values was in accordance with the studies conducted by Mehta AB et al., Zhang Q et al., Lata S et al., Shetty S et al., and Neto FCR et al., [2,7,11,15,32]. Lata S et al., reported that initial enamel lesions with intact surfaces record a low mineral content at the surface layer when compared to sound enamel; thus, demonstrating a lower microhardness value at the surface than for sound enamel tissue [11].
The experimental remineralising agents were applied topically to the enamel specimens twice a day for a period of two minutes each, to simulate the normal recommended daily oral prophylaxis. Various studies have performed the pH cycling process at different lengths of time ranging from 7-14 days. Balakrishnan A et al., evaluated the remineralisation potential of various dentifrices over a period of 30 days and concluded that the extent of remineralisation achieved was dose dependant and increased with increasing the time of exposure and duration of the study [22]. Therefore, in the current study, the pH cycling process was carried out for 30 days.
At the conclusion of 30 days, SMH evaluation was carried out. An increase in mean microhardness was observed in all the groups. The remineralisation values exhibited statistically significant differences between the groups [Table/Fig-2,3].
The use of fluoride is an effective method for promoting the remineralisation of early enamel lesions through the formation of fluorapatite. However, for every two fluoride ions, ten calcium ions and six phosphate ions are required to form one unit cell of fluorapatite {Ca10(PO4)6F2}. Hence, when topically applying fluoride, an inadequate amount of available calcium and phosphate ions can limit net enamel remineralisation. CPP-ACP has demonstrated superior properties in situ in terms of anticariogenic activity, increasing levels of calcium and phosphate ions significantly in supragingival plaque, and promoting the remineralisation of enamel subsurface lesions [12]. The synergistic effect of CPP-ACP and fluoride in reducing caries may be attributable to the formation of CPP-stabilized amorphous calcium fluoride phosphate, resulting in the increased incorporation of fluoride ions into plaque, together with elevated concentrations of bioavailable calcium and phosphate ions [14,37-40].
Reynolds EC et al., reported that CPP–ACPF has a greater potential for remineralisation than CPP–ACP [12]. Hence, in the present study CPP-ACPF was compared with the other agents. The remineralising potential of CPP-ACPF was significantly greater than fluoride enhanced HA gel. CPP-ACPF performed marginally better than BAG; however no significant difference was noted between the two groups, which is in accordance with the findings of Balakrishnan A et al., and Neto FCR et al., [22,32].
BAG is an extensively studied biomaterial in the field of tissue engineering, bone regeneration and dentin remineralisation due to the remarkable capability of forming Hydroxycarbonate Apatite (HCA) [25,14]. Bioactive glass 45S5 (BAG) has been incorporated into dentifrices, desensitizing pastes and glass ionomer cements (experimentally). Although, it has been successfully proven that materials based on bioactive substance have the potential to promote remineralisation, only a limited number of studies have quantitatively monitored the remineralisation process [14,41].
It has been reported that, when BAG comes in contact with saliva or any aqueous media, its active ingredient, calcium sodium phosphosilicate, binds to the tooth surface in order to initiate the remineralisation process. The BAG thereby reacts with saliva inducing dissolution of calcium, phosphate and silicate ions at the glass surface and subsequent precipitation of a polycondensed silica-rich layer which serves as a template for the formation of calcium phosphate which subsequently crystallise into HCA [24,38,14].
The results of the current study revealed an increase in SMH after remineralisation with BAG. This could be attributed to the precipitation of a HCA layer on the surface of the enamel. Although, there was no significant difference between CPP-ACPF and BAG, BAG remineralised enamel less effectively as compared to CPP-ACPF, which was in agreement with the findings of Preethee T et al., [42].
There are limited in vitro studies evaluating the remineralising efficacy of fluoride enhanced HA gel. Heshmat H et al., and Kamath U et al., reported no difference between CPP-ACPF and fluoride enhanced HA gel [26,27]. The authors hypothesised that the synergistic action of the HA and fluoride, enhanced remineralisation thereby rendering the tooth more resistant to acid attacks. In contrast, the present in vitro investigation revealed a statistically significant difference between CPP-ACPF and fluoride enhanced HA gel, with CPP-ACPF exhibiting superior remineralising property which could be attributed to the characteristic nature of CPP. CPP by stabilizing calcium phosphate in a metastable solution facilitates increased concentrations of calcium and phosphate ions, including dicalcium phosphate (CaHPO4), which can diffuse into the enamel subsurface lesion, while the fluoride synergistically enhances remineralisation [22].
Research has established that self-assembling peptides undergo self-assembly into three-dimensional (3D) fibrillar scaffolds in response to specific environmental triggers. At certain peptide concentrations, P11-4 switches from a low viscosity isotropic liquid to an elastomeric nematic gel at pH <7.4, the anionic groups of the P11-4 side chains then attract calcium ions, activating precipitation of new hydroxyapatite, thereby promotingmineral deposition in situ. P11-4 is a bioactive peptide synthesised from natural amino acids that is triggered to assemble into a 3D scaffold by environmental fluctuations of pH and salt concentration. This organisation occurs within the lesion, and the scaffold can then function as nucleator for hydroxyapatite, inducing tissue regeneration from within [28,29].
The results of the clinical trial conducted by Brunton PA et al., suggested that treatment of early caries lesions with P11-4 is safe, and a single application is associated with significant enamel regeneration, presumably by promoting mineral deposition within the subsurface tissue [28]. Kirkham J et al., showed that P11-4 is able to nucleate new hydroxyapatite crystals and promote repair of caries like lesions in vitro [29]. While Jablonski-Momeni A et al., concluded that the SEM images of samples treated with self-assembling peptide P11-4 revealed large areas of remineralised enamel surface in 93 % of the samples, thereby proving to be efficacious [43].
In the current investigation, the self assembling peptide P11-4 group showed the best results, with considerably greater increase in the percentage of SMH recovery as compared to the other groups [Table/Fig-4]. This could be attributed to the ability of the peptide to induce biomimetic mineralisation by nucleating hydroxyapatite crystals.
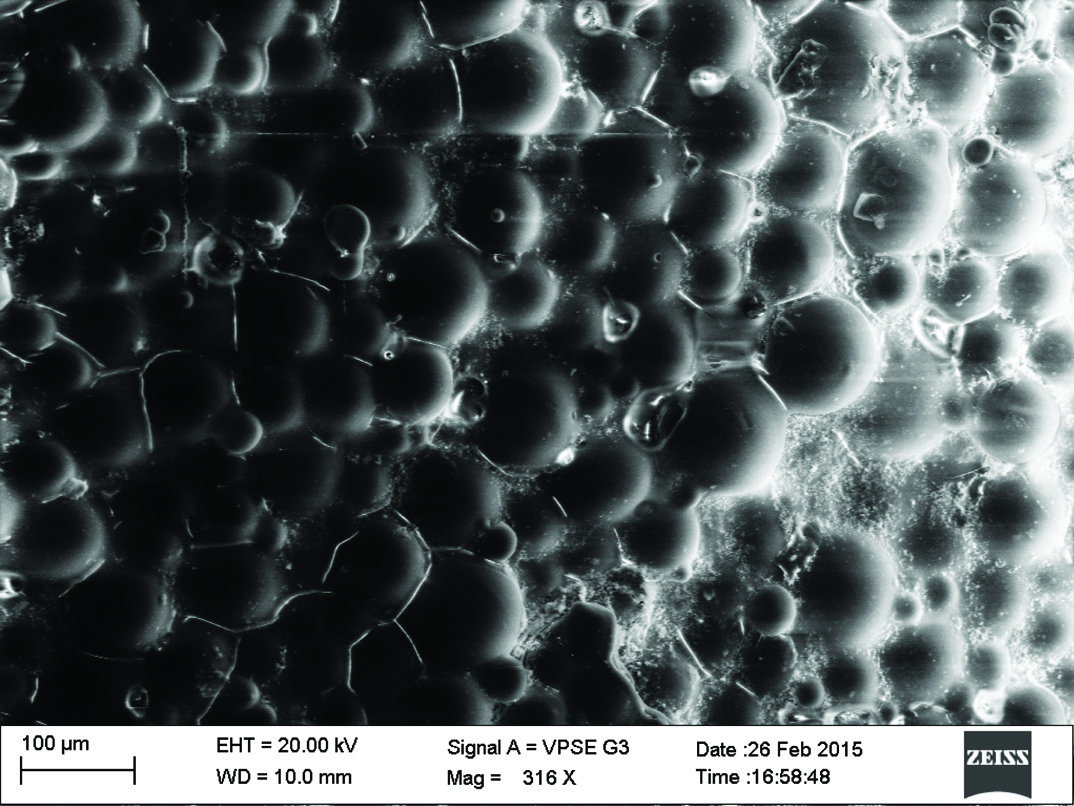
Qualitative assessment was carried out using SEM analysis. SEM images of the sound enamel showed well organised enamel rods [Table/Fig-5]. The enamel crystals were homogeneously arranged with a clear outline. In contrast, the demineralised enamel was disorganized, with loss of structural characteristics [Table/Fig-6]. All the test groups demonstrated either amorphous crystals or particles scattered on the surface or lines of remineralisation along the prismatic borders [Table/Fig-7,8,9 and 10].
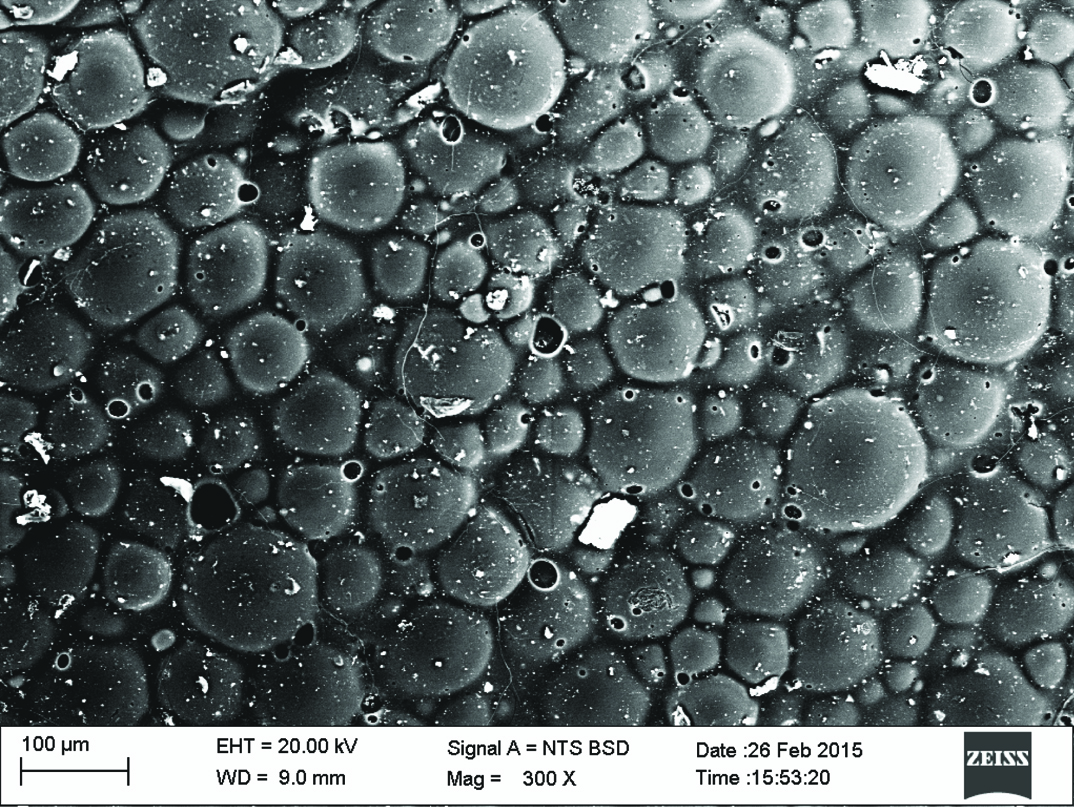
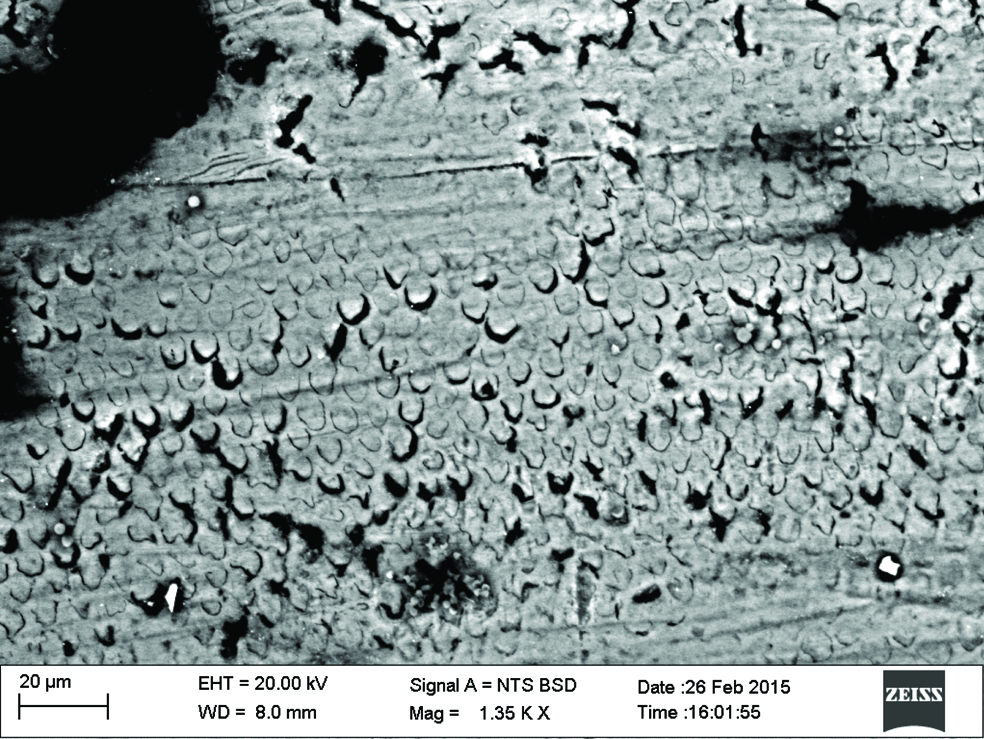
CPP-ACPF group. (Images left to right)
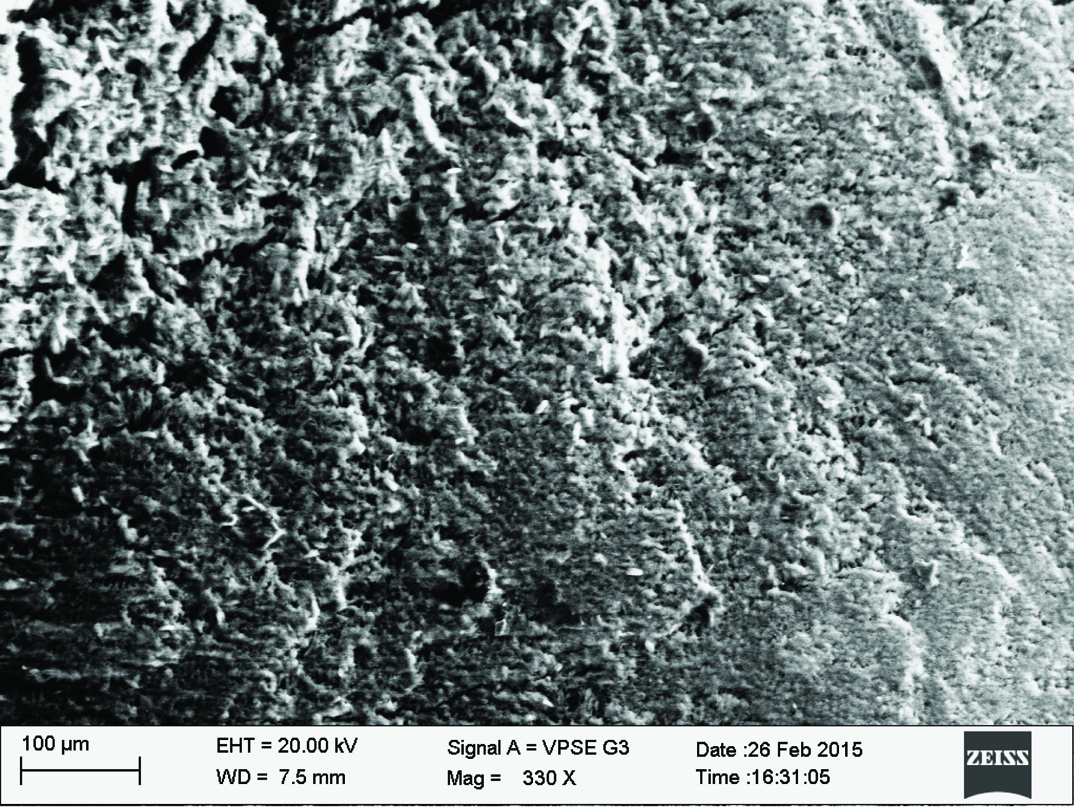
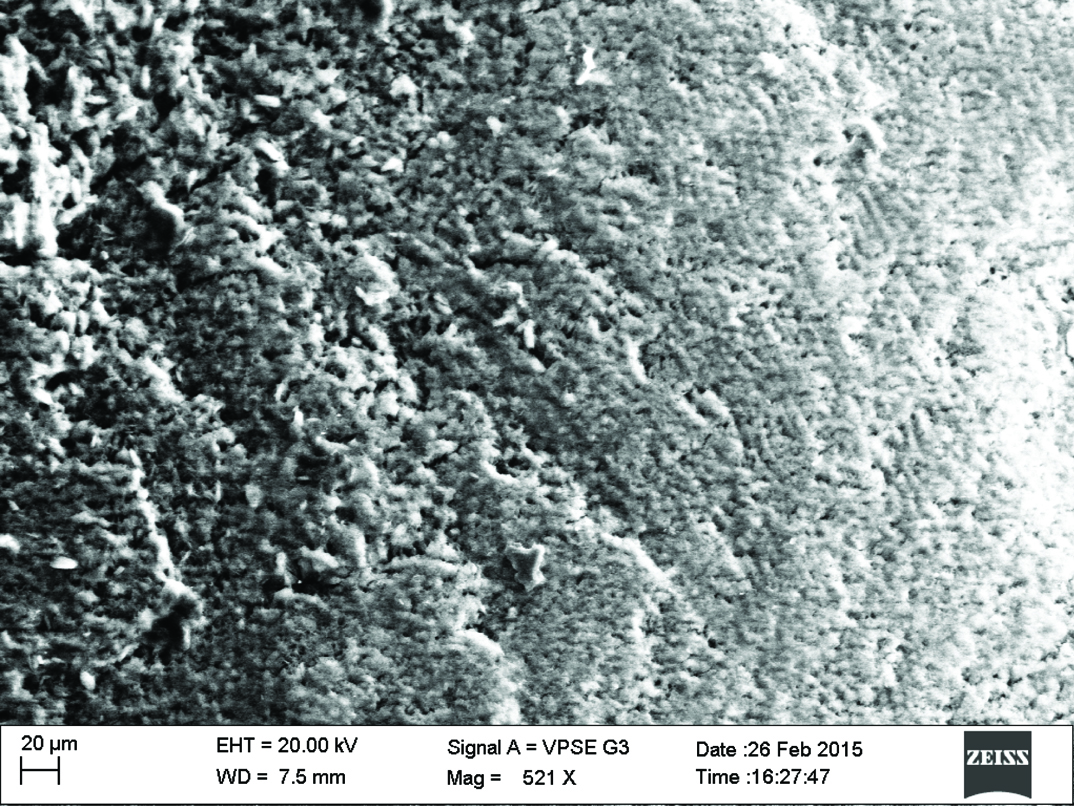
Fluoride enhanced hydroxyapatite gel group.
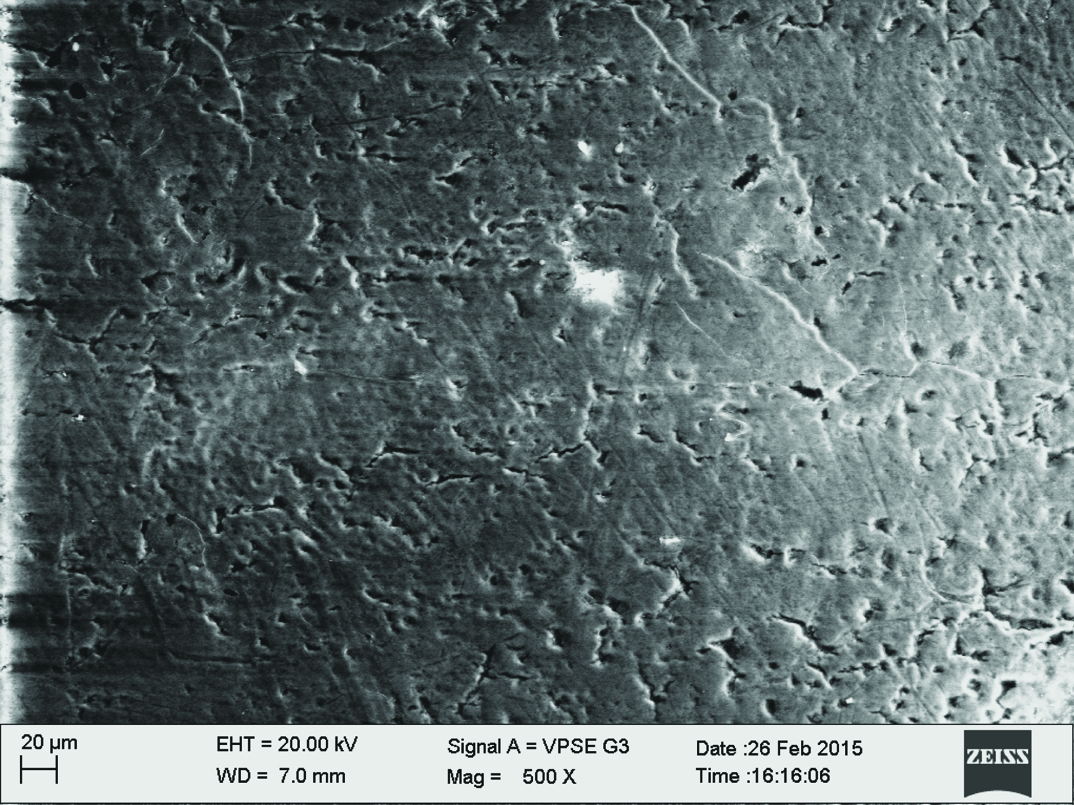
Self assembling peptide P11-4 group.
Limitation
The limitations of this in vitro study include difficulty to precisely simulate the biological aspects of caries and the multitude of intraoral conditions that contribute to dental caries, the role of enzymes is not accounted for. Since solutions are composed of inorganic ions only, the effects of salivary proteins, pellicle and plaque on mineralisation inhibition are not taken into consideration. Other confounding factors involve the possibility of experimental errors and dissimilarities in the micro-structure of the enamel between specimens.
Conclusion
Within the limitations of the present study, it can be concluded that, self assembling peptide P11-4 exhibited a significant difference in remineralising enamel lesions. Although, no significant difference was observed between the P11-4 and CPP-ACPF group, the self assembling peptide remineralised the enamel lesions more effectively. The remineralising potential demonstrated by self assembling peptide P11-4 was observed to be the highest followed by CPP-ACPF, BAG and fluoride enhanced HA gel.
It is imperative to note that remineralisation in vitro may be quite variable when compared to changes occurring in the oral cavity in vivo. Therefore, direct extrapolations to clinical situations must be executed discreetly.
Post-Hoc Tukey Test
*. The mean difference is significant at the 0.05 level.
CPP-ACPF, Casein phosphopeptide amorphous calcium phosphate fluoride; BAG, Bioactive Glass; FEHG, Fluoride enhanced hydroxyapatite gel; P11-4, Self-Assembling Peptide P11-4.