A Retrospective Study to Assess the Effect of Proton Pump Inhibitors on Renal Profile in a South Indian Hospital
A. Avinash1, Navin Patil2, Sushil Kiran Kunder3, O. Balaji4, Amod Tilak5, Ravi K. Sori6, Raghavendra Rao7
1 Postgraduate Student, Department of Pharmacology, Kasturba Medical College, Manipal Universty, Manipal, Karnataka, India.
2 Assistant Professor, Department of Pharmacology, Kasturba Medical College, Manipal Universty, Manipal, Karnataka, India.
3 Postgraduate Student, Department of Pharmacology, Kasturba Medical College, Manipal Universty, Manipal, Karnataka, India.
4 Postgraduate Student, Department of Pharmacology, Kasturba Medical College, Manipal Universty, Manipal, Karnataka, India.
5 Postgraduate Student, Department of Pharmacology, Kasturba Medical College, Manipal Universty, Manipal, Karnataka, India.
6 Postgraduate Student, Department of Pharmacology, Kasturba Medical College, Manipal Universty, Manipal, Karnataka, India.
7 Assistant Professor, Department of Medicine, Kasturba Medical College, Manipal Universty, Manipal, Karnataka, India.
NAME, ADDRESS, E-MAIL ID OF THE CORRESPONDING AUTHOR: Dr. Raghavendra Rao, Assistant Professor, Department of Medicine, Kasturba Medical College, Manipal Universty, Manipal-576104, Karnataka, India.
E-mail: ragsmanipal1983@gmail.com
Introduction
Proton Pump Inhibitors (PPIs) are arguably among the most commonly prescribed drugs in clinical practice, either as part of treatment or prophylaxis. Many clinicians prescribe these drugs as part of any prescription, without a proper rationale. Recent studies done outside India have shown that these drugs are not entirely safe, and they can result in the development of acute renal injury.
Aim
To assess the effect of PPIs on blood urea and serum creatinine, when administered for at least seven consecutive days.
Materials and Methods
The study was conducted in a retrospective manner, using data from the medical records department. Values of blood urea and serum creatinine were taken twice, first before start of therapy and then after at least one week of therapy.
Results
A total of 175 subjects were selected for the study. When their case files were analysed, acute kidney injury was identified in 19 (10.86%) of them. Pantoprazole was the most common drug involved (84.21%). Renal injury was more common in the age group of over 50 years of age.
Conclusion
PPIs are not entirely free of adverse effects, as assumed by several practitioners. A vigilant eye has to be maintained on the patient’s renal profile so as to avoid any untoward decline in renal function, as evidenced in the current study.
Creatinine, Omeprazole, Pantoprazole, Urea
Introduction
PPIs are a group of drugs commonly prescribed in the management and prophylaxis of acid peptic disorders. They act by inhibiting the H+-K+-ATPase enzyme (proton pump) present in the parietal cells of the gastric mucosa. These drugs are the most potent blockers of gastric acid secretion, as they block the secretion irreversibly. Their efficacy has been estimated to be better than that of histamine-2 receptor blockers [1,2]. PPIs are also recommended for prophylaxis of peptic ulcer in NSAID users, eradication of Helicobacter pylori-related ulcers and also for the management of Gastroesophageal Reflux Disease (GERD). Pantoprazole is arguably the most commonly prescribed PPI. Rabeprazole, omeprazole and lansoprazole are few of the other PPIs that are routinely prescribed around the world [3-5].
PPIs have a very good safety profile, as a result of which they are commonly prescribed by healthcare providers throughout the world. In 2009, it has been estimated that over 119 million prescriptions contained one or the other PPI in the USA. However, they are not entirely free of adverse events. The rate of incidence of adverse effects is estimated to be about 1 to 3%. The adverse effect profile includes headache, abdominal pain, nausea, constipation, diarrhoea, flatulence and rashes. Prescription of prolonged courses of PPIs is becoming increasingly common, at times even without the right indications, owing to their projected safe nature [6]. Occasionally, the patients consume PPIs for long periods, as Over-The-Counter (OTC) medications. Such long-term use of PPIs is a cause of concern as there has been increasing evidence of several adverse effects. Nutritional deficiencies like B12 and iron deficiency, hypomagnesemia and hypocalcaemia have been reported. Fractures (possibly secondary to hypocalcaemia), respiratory and enteric infections, hypergastrinemia, gastric polyps and gastric cancer are well known adverse effects of persistent use of PPIs. Despite the exhausting list of adverse effects, PPIs are still being widely prescribed and used because most of these adverse effects present themselves in a mild nature, and hence are usually clinically insignificant [7].
Kidney injury associated with PPIs has gained limelight in recent times. Both acute and chronic varieties have been listed to be adverse effects of long term PPI usage. Acute Kidney Injury (AKI) is said to have set in, when the glomerular filtration rate declines rapidly, which causes the nitrogen based waste products to get accumulated in the body. This is evidenced by an increase in the levels of blood urea nitrogen and serum creatinine. Although these biomarkers are not very specific, they are commonly used to determine whether a patient has developed AKI or not, in the Indian setup, irrespective of the cause of AKI [8].
Two recent studies (a population based cohort study and a nested case-control study) concluded that PPI therapy is associated with an increased risk of developing AKI [9,10]. In addition, a recent population based cohort study suggested that use of PPIs for long periods increases the chances of developing Chronic Kidney Disease (CKD) [11]. However, no such studies have been done in the Indian population.
Hence, the main intention of our current study was to assess the effect of PPIs on renal profile and to estimate the rate of incidence of AKI associated with the use of PPIs, in the south Indian population, using laboratory values of blood urea and serum creatinine, before and after at least one week of therapy.
Materials and Methods
The study was a retrospective hospital based analysis, done in the Medical Records Department of Kasturba Hospital, Manipal, India. The study was carried out after ethical clearance was obtained from the Institutional Ethics Committee (Ref. no. IEC/687/2016). The data was collected by the authors from October 2016 to December 2016 (around two months).
Inclusion Criteria
Patients aged at least 18 years, of both sexes;
Patients who were outpatients or inpatients in the Department of Internal Medicine, Kasturba Hospital, Manipal from January 2015 to December 2015, and were on PPIs for at least one week (seven consecutive days) for treatment or prophylaxis of gastritis, duodenitis or oesophagitis due to any cause;
Patients whose medical records contained at least one renal profile measurement before, and after at least one week of therapy with PPIs. One week was chosen as the minimal cut off period based on previous definitions of AKI [12]. Patients who used PPIs for long periods beyond one week were also included in the study.
Exclusion Criteria
Patients who had abnormally elevated laboratory renal parameters (urea and creatinine) prior to initiation of PPI therapy;
Patients who had a history of having undergone dialysis or renal transplant before or during the PPI therapy;
Pregnant and lactating women;
Patients who were concomitantly administered known nephrotoxic drugs.
All patients who were diagnosed to have gastritis, duodenitis or oesophagitis by the Department of Internal Medicine between January 2015 and December 2015 (a time based study over a period of one year), and also fulfilled the selection criteria were taken into consideration for the study. A total of 450 patients had visited the Department of Internal Medicine in the year of 2015. All of their case records were identified and examined for satisfaction of selection criteria. 175 case records fulfilled the selection criteria, and were included in our study.
Procedures
Case records of all selected patients were obtained from the Medical Records Department and analysed for the following parameters:
Type of PPI used;
Duration and dosage of PPI therapy;
Values of blood urea and serum creatinine before and after therapy with PPIs.
The clinical diagnoses and symptoms of AKI that the patients might have had, have not been considered in the study. Only the laboratory based biochemical criteria (urea and creatinine levels) were used. This was done because it was a retrospective case record based study, which makes it highly likely that the symptoms might not be available in the case records. However, all laboratory parameters in this hospital are digitalized and stored in the online database, thus making them a better choice for retrospective analyses.
Statistical Analysis
The data obtained were tabulated and analysed using IBM SPSS version 21.0.
Results
A total of 450 case files were screened for eligibility. All these 450 patients were chosen based on their final diagnosis (gastritis/duodenitis/oesophagitis due to any cause). Out of these 450 cases, 175 cases satisfied the selection criteria and the others were eliminated for various reasons, as shown in [Table/Fig-1].
Distribution of the study population.
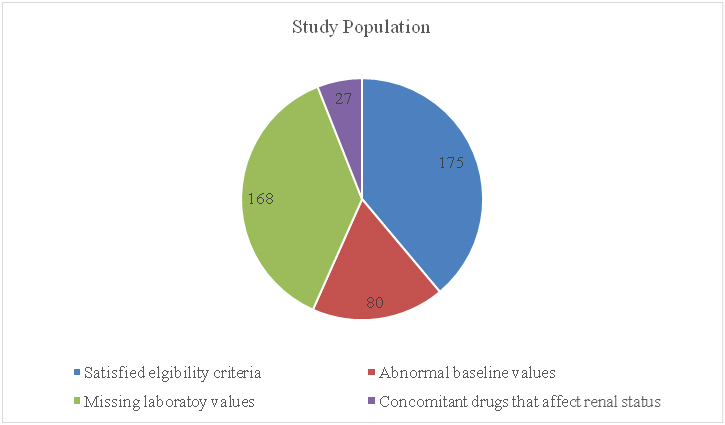
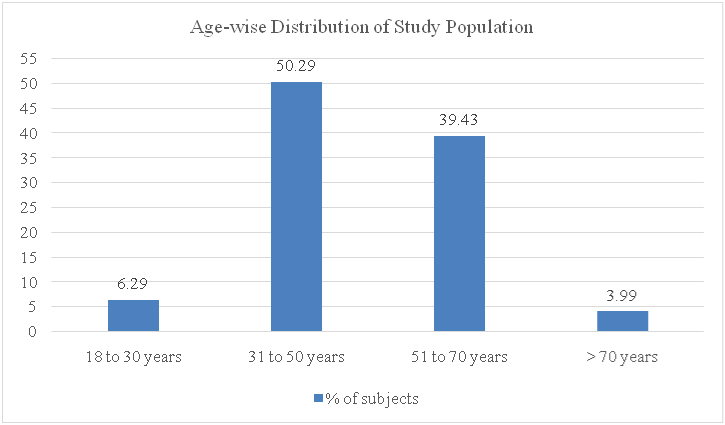
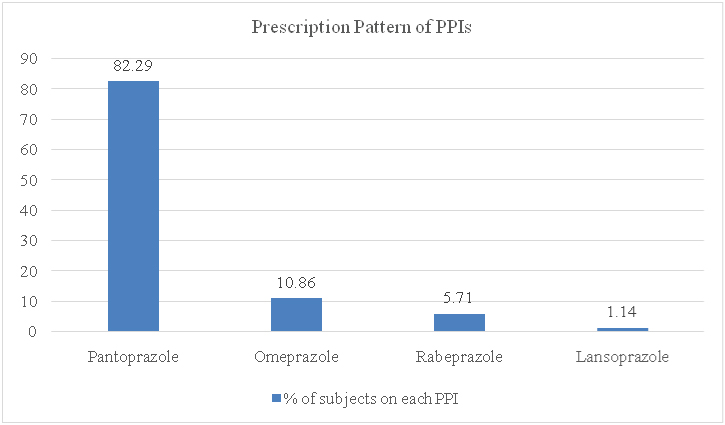
As shown, 175 case files were analysed for further data in our study. Out of these 175 cases, 106 (60.57%) were males and 69 (39.43%) were females. All cases were adults, as per the selection criteria. The age wise distribution of the study population was as shown in [Table/Fig-2]. Majority of the subjects fell in the age group of 31 to 50 years. Pantoprazole was the most common PPI prescribed among the 175 cases, followed by omeprazole and rabeprazole. Lansoprazole was used in only two patients. No other PPIs were used by the subjects in the study. [Table/Fig-3] depicts the prescription pattern of PPIs in the study population.
Age wise distribution of the study subjects (age group of the patients in the X-axis plotted against the percentage of subjects in the Y-axis).
Distribution of PPIs among the study population (the type of PPI on the X-axis plotted against the percentage of subjects in the Y-axis).
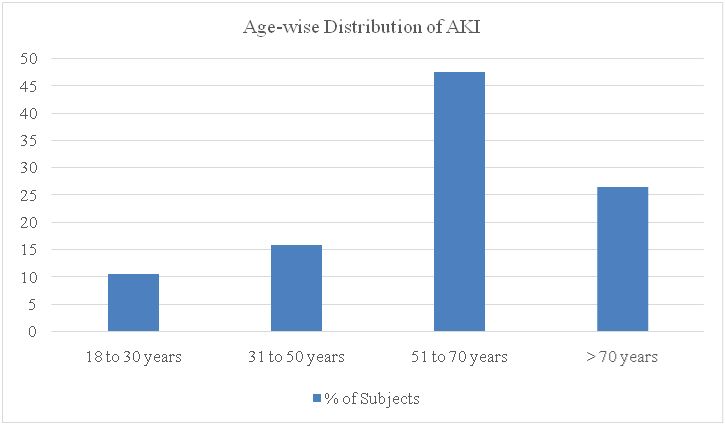
AKI occurred in 19 (10.86%) subjects out of the 175 analysed. The age wise distribution of the incidence of AKI is shown in [Table/Fig-4]. The incidence of AKI was more in the population over 50 years, with the maximal incidence in the group of 51 to 70 years. Of the 19 subjects who developed AKI, 15 (78.95%) were males, and 4 (21.05%) were females.
Age-wise distribution of the incidence of acute kidney injury (Age groups on the X-axis plotted against the percentage of subjects in the Y-axis).
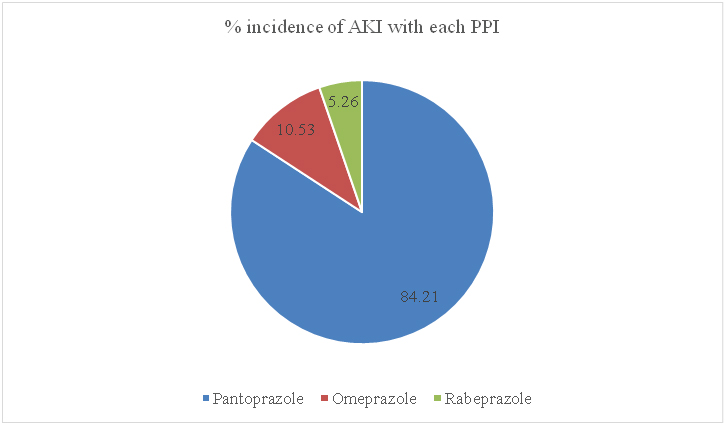
Pantoprazole was the most common cause among the PPIs causing AKI, followed by omeprazole and rabeprazole. Lansoprazole was not found to cause AKI in either of the two patients in whom it was prescribed. The distribution is depicted in [Table/Fig-5].
Percentage of incidence of acute kidney injury with each PPI.
[Table/Fig-6] depicts the baseline and end of study biochemical values of the patients who developed AKI following PPI therapy. The normal values in our laboratory are as follows: blood urea; 10 to 40 mg/dl; serum creatinine; 0.4 to 1.2 mg/dl.
Biochemical laboratory values of the patients who developed AKI (All values are expressed as mg/dl).
| S.No. | PPI used | Duration ofPPI therapy(in days) | Baseline values | Values at theend of PPItherapy |
|---|
| Bloodurea | Serumcreatinine | Bloodurea | Serumcreatinine |
|---|
| 1 | Pantoprazole | 11 | 32 | 0.7 | 66 | 1.8 |
| 2 | Pantoprazole | 9 | 26 | 1.0 | 30 | 1.4 |
| 3 | Pantoprazole | 18 | 18 | 1.1 | 30 | 1.4 |
| 4 | Pantoprazole | 12 | 11 | 1.0 | 35 | 1.6 |
| 5 | Pantoprazole | 14 | 31 | 0.9 | 54 | 1.7 |
| 6 | Pantoprazole | 8 | 11 | 0.9 | 45 | 2.0 |
| 7 | Pantoprazole | 21 | 30 | 1.2 | 44 | 1.6 |
| 8 | Pantoprazole | 16 | 25 | 1.1 | 54 | 1.9 |
| 9 | Omeprazole | 21 | 29 | 0.4 | 68 | 1.4 |
| 10 | Pantoprazole | 14 | 10 | 0.7 | 120 | 3.4 |
| 11 | Pantoprazole | 14 | 32 | 0.6 | 46 | 1.4 |
| 12 | Pantoprazole | 7 | 25 | 1.1 | 77 | 1.5 |
| 13 | Pantoprazole | 21 | 10 | 0.7 | 41 | 1.7 |
| 14 | Pantoprazole | 14 | 21 | 0.8 | 42 | 1.3 |
| 15 | Rabeprazole | 14 | 37 | 1.1 | 69 | 1.6 |
| 16 | Pantoprazole | 7 | 34 | 0.6 | 65 | 1.4 |
| 17 | Omeprazole | 14 | 37 | 1.2 | 46 | 2.9 |
| 18 | Pantoprazole | 18 | 39 | 0.9 | 77 | 1.4 |
| 19 | Pantoprazole | 7 | 18 | 0.8 | 43 | 1.5 |
No relationship was found to be present between the duration of PPI therapy and the onset of AKI. No information was available in the case records regarding the causality assessment of each subject. However, the authors performed a causality assessment, which showed that 12 of these subjects had “possible” and seven had “probable” causality. In none of the 19 subjects, the PPI therapy was stopped due to development of AKI. In nine of the patients, intravenous fluids were administered as treatment, following the onset of AKI. There were no fatalities in the study. All 19 patients recovered from AKI.
Discussion
Around 20% of the cases of AKI are said to be drug induced [13]. Although several drugs have been reported to cause AKI, the pharmacological class of PPIs has rarely made it to the list of such drugs [14]. However, recent evidence suggests otherwise. In the current study, 19 subjects were found to have developed AKI among the 175 patient records assessed. Thus, an incidence of 10.86% was estimated in the study. An incidence of 2.86% (6 cases out of 210) was found in a study done in Southeast England. The lesser incidence could be because the study considered only biopsy positive cases of Acute Interstitial Nephritis (AIN) [15].
Although the study population was predominantly in the age group of 31 to 50 years of age, the incidence of AKI was more common in the group of 51 to 70 years of age, probably relating the development of AKI to the physiological decline in renal functions. However, a case series analysis done in the Netherlands shows no such age or gender bias [16]. Another case series from New Zealand showed the median age to be 78 years (more common in the elderly) [17].
Pantoprazole was the most common PPI responsible for the development of AKI in the current study but this could be because 82% of the study population had been prescribed pantoprazole, as opposed to the other PPIs. A study by Sampathkumar K et al., also showed that pantoprazole was the most commonly implicated PPI [18]. However, there are several reports available, which report AKI with other PPIs [19-23]. AKI is now considered as a class effect of PPIs [24]. The dosage and duration of PPI therapy did not play a significant role in the development of AKI. Similar results were observed in previous studies as well [16].
The duration of PPI therapy preceding the development of AKI varied from 7 to 21 days in the current study. The available literature shows a varied range, from one week to three months [18,25].
The exact mechanism behind this adverse effect is unclear. Several hypotheses have been put forward. The AIN seen with PPIs is probably the result of an immune response to the exogenously administered drug. Activation and expression of nuclear factor kappa B have been implicated in the pathogenesis [26].
Also, it has been postulated that AKI could be secondary to oxidative stress, which could be identified with the use of biomarkers like vanin-1. Urinary vanin-1 levels could pose as a prospective biomarker to detect AKI [27].
Further, the hypomagnesemic component associated with the use of PPIs could be another reason for development of AKI and CKD, especially in the older population [28].
Limitation
The current study was performed in a retrospective manner. Hence, case files that did not have the required lab investigation reports were excluded, which reduced the sample size of the study. Only cases who were on PPI treatment for gastritis, oesophagitis and duodenitis were considered for the study. Pantoprazole was the most commonly prescribed PPI in our hospital, which could have created a bias in the form of increased incidence of AKI with the usage of pantoprazole.
Conclusion
Although PPIs are being prescribed routinely for both treatment and for prophylaxis, they are not entirely safe. The adverse effect on the patients’ renal profile should always be kept in mind by the physicians. Patients who are at higher risk of developing renal failure should be even closely monitored. Further studies are required in the Indian population with larger sample size, to know the exact impact of PPIs on the renal profile, and also to assess the causality of AKI in such patient populations.
[1]. Gisbert JP, Gonzalez L, Calvet X, Roque M, Gabriel R, Pajares JM, Proton pump inhibitors versus H2-antagonists: a meta-analysis of their efficacy in treating bleeding peptic ulcerAliment Pharmacol Ther 2001 15:917-26. [Google Scholar]
[2]. Eriksson S, Langstrom G, Rikner L, Carlsson R, Naesdal J, Omeprazole and H2-receptor antagonists in the acute treatment of duodenal ulcer, gastric ulcer and reflux oesophagitis: a metaanalysisEur J Gastroenterol Hepatol 1995 7:467-75. [Google Scholar]
[3]. Lanza FL, Chan FK, Quigley EM, Guidelines for prevention of NSAID-related ulcer complicationsAm J Gastroenterol 2009 104:728-38. [Google Scholar]
[4]. Malfertheiner P, Megraud F, O’Morain C, Bazzoli F, El-Omar E, Graham D, Current concepts in the management of Helicobacter pylori infection: the Maastricht III consensus reportGut 2007 56(6):772-81. [Google Scholar]
[5]. DeVault KR, Castell DO, Updated guidelines for the diagnosis and treatment of gastroesophageal reflux diseaseAm J Gastroenterol 2005 100:190-200. [Google Scholar]
[6]. Ali T, Roberts DN, Tierney WM, Long-term safety concerns with proton pump inhibitorsAm J Med 2009 122:896-903. [Google Scholar]
[7]. Sheen E, Triadafilopoulos G, Adverse effects of long-term proton pump inhibitor therapyDig Dis Sci 2011 56:931-50. [Google Scholar]
[8]. Usha Gupta T, Prakash J, Prakash S, Rathore SS, Sunder S, Acute kidney injury in patients with human immunodeficiency virus infectionIndian J Nephrol 2015 25(2):86-90. [Google Scholar]
[9]. Antoniou T, Macdonald EM, Hollands S, Gomes T, Mamdani MM, Garg AX, Proton pump inhibitors and the risk of acute kidney injury in older patients: a population-based cohort studyCMAJ Open 2015 3(2):E166-71. [Google Scholar]
[10]. Klesper DG, Collier DS, Cochran DR, Proton pump inhibitors and acute kidney injury: a nested case–control studyBMC Nephrol 2013 14:150 [Google Scholar]
[11]. Lazarus B, Chen Y, Wilson FP, Sang Y, Chang AR, Coresh J, Proton pump inhibitor use and the risk of chronic kidney diseaseJAMA Intern Med 2016 176(2):238-46. [Google Scholar]
[12]. The Renal Association guidelines 2011. Available from http://www.renal.org/guidelines/modules/acute-kidney-injury#sthash.e2IYjVMW.dpbs (Last accessed on 27th December, 2016 at 15:00 hours) [Google Scholar]
[13]. Naughton CA, Drug-induced nephrotoxicityAm Fam Physician 2008 78(6):743-50. [Google Scholar]
[14]. Yokoyama H, Narita I, Sugiyama H, Nagata M, Sato H, Ueda Y, Drug-induced kidney disease: a study of the Japan Renal Biopsy Registry from 2007 to 2015Clin Exp Nephrol 2016 20:720-30. [Google Scholar]
[15]. Ray S, Delaney M, Muller AF, Proton pump inhibitors and acute interstitial nephritisBMJ 2010 341:c4412 [Google Scholar]
[16]. Hamark L, van der Wiel HE, de Groot MC, van Grootheest AC, Proton pump inhibitor-induced acute interstitial nephritisBr J Clin Pharmacol 2007 64(6):819-23. [Google Scholar]
[17]. Simpson IJ, Marshall MR, Pilmore H, Manley P, Williams L, Thein H, Proton pump inhibitors and acute interstitial nephritis: report and analysis of 15 casesNephrology (Carlton) 2006 11(5):381-85. [Google Scholar]
[18]. Sampathkumar K, Ramalingam R, Prabakar A, Abraham A, Acute interstitial nephritis due to proton pump inhibitorsIndian J Nephrol 2013 23(4):304-07. [Google Scholar]
[19]. Moore I, Sayer JA, Nayar A, Ahmed S, Tapson JS, Pantoprazole-induced acute interstitial nephritisJ Nephrol 2004 17(4):580-81. [Google Scholar]
[20]. Geevasinga N, Coleman PL, Roger SD, Rabeprazole-induced acute interstitial nephritisNephrology (Carlton) 2005 10(1):07-09. [Google Scholar]
[21]. D’Adamo G, Spinelli C, Forte F, Gangeri F, Omeprazole-induced acute interstitial nephritisRen Fail 1997 19(1):171-75. [Google Scholar]
[22]. Geevasinga N, Coleman PL, Webster AC, Roger SD, Proton pump inhibitors and acute interstitial nephritisClin Gastroenterol Hepatol 2006 4(5):597-604. [Google Scholar]
[23]. Blank ML, Parkin L, Paul C, Herbison P, A nationwide nested case-control study indicates an increased risk of acute interstitial nephritis with proton pump inhibitor useKidney Int 2014 86:837-44. [Google Scholar]
[24]. Brewster UC, Perazella MA, Proton pump inhibitors and the kidney: critical reviewClin Nephrol 2007 68(2):65-72. [Google Scholar]
[25]. Myers RP, McLaughlin K, Hollomby DJ, Acute interstitial nephritis due to omeprazoleAm J Gastroenterol 2001 96:3428-31. [Google Scholar]
[26]. Hosohata K, Role of oxidative stress in drug-induced kidney injuryInt J Mol Sci 2016 17:1826-35. [Google Scholar]
[27]. Hosohata K, Ando H, Fujimura A, Urinary Vanin-1 as a novel biomarker for early detection of drug-induced acute kidney injuryJ Pharmacol Exp Ther 2012 341:656-62. [Google Scholar]
[28]. Peng Y, Lin C, Yeh H, Chang C, Wu Y, Kao C, Association between the use of proton pump inhibitors and the risk of ESRD in renal diseases: a population-based, case-control studyMedicine 2016 95(15):e3363 [Google Scholar]