Role of Echocardiography in Prenatal Screening of Congenital Heart Diseases and its Correlation with Postnatal Outcome
Shivani Sharma1, Navkiran Kaur2, Khushpreet Kaur3, Naveen Chandrashekhar Pawar4
1 Junior Resident, Department Radiology, Government Medical College and Rajindra Hospital, Patiala, Punjab, India.
2 Professor and Head, Department of Radiology, Government Medical College and Rajindra Hospital, Patiala, Punjab, India.
3 Professor and Head, Department of Obstetrics and Gynecology, Government Medical College and Rajindra Hospital, Patiala, Punjab, India.
4 Junior Resident, Department of Radiology, Government Medical College and Rajindra Hospital, Patiala, Punjab, India.
NAME, ADDRESS, E-MAIL ID OF THE CORRESPONDING AUTHOR: Dr. Shivani Sharma, Junior Resident, Department of Radiology, Rajindra Hospital and Government Medical College, Patiala-147001, Punjab, India.
E-mail: highfive7755@gmail.com
Introduction
Congenital Heart Defects (CHDs) are one of the most common forms of congenital anomalies. Fetal echocardiography performed during second trimester aims at early diagnosis of congenital heart disease which is instrumental in proper planning of delivery, perinatal care and counselling of parents.
Aim
To evaluate the role of fetal echocardiography in prenatal screening of CHDs and to study the role of associated extracardiac anomalies.
Materials and Methods
This was a hospital based prospective and correlative type of study, done over a period of one year. Antenatal screening of fetal heart was done in mid-trimester high and low risk pregnancies. The prenatal echo findings were co-related with postnatal findings in case of any abnormality detected. The extra-cardiac anomalies associated with positive cases were evaluated and studied for their impact on postnatal outcome.
Results
A total of 1200 pregnancies were screened out of which 672 were low risk and 528 were high risk. The cases with abnormal echo findings were followed postnatally. The overall incidence of CHD in study population was 15 per 1000. The incidence in high and low risk pregnancies were 16.3 and 13.25 per thousand respectively. Complete agreement of 68.17% was found between prenatal and postnatal findings. The most frequent Extra-Cardiac Anomalies (ECA) in cases with CHD was of musculoskeletal system. The CHD cases with ECA were significantly of low birth weight, born preterm and delivered by Lower Segment Caesarean Section (LSCS).
Conclusion
Fetal heart is the most overlooked part in every routine anomaly scan. We conclude that fetal echocardiography should be an integral part of every second trimester anomaly scan for all pregnant females irrespective of their risk factors. The associated ECAs are another factor that causes increased mortality both in antenatal and neonatal life, again warranting an early fetal echo.
Extra-cardiac anomalies, Fetal heart, Second trimester
Introduction
Structural abnormalities of the heart and great vessels are the most common severe congenital abnormalities, with a prevalence of thee to eight per 1000 live births [1-3]. Studies have shown that CHDs are six times more common than chromosomal abnormalities and four times more common than neural tube defects [4]. However, due to the complex cardiac anatomy and dynamic nature, CHDs especially the small Ventricular Septal Defects (VSDs) often go undetected during antenatal scans [5].
There are a number of risk factors associated with CHDs which might be either maternal or fetal. The maternal risk factors include diabetes, autoimmune disorders like systemic lupus erythematosus or Sjogren’s syndrome, use of drugs, e.g., antiepileptics or antipsychotics like lithium, etc., CHD in the mother; whereas, the fetal risk factors are infections, history of a sibling with a cardiac anomaly, prominent nuchal translucency or increased nuchal fold thickness, structural defect in other systems, Intrauterine Growth Retardation (IUGR) in the mid-trimester [6].
Extracardiac abnormalities are frequently seen in patients with congenital heart disease and patients with these alterations may present an increased risk of morbidity and mortality [7]. Levi et al., concluded that Associated Congenital Heart Diseases (ACHDs) were associated with increased mortality, preterm births and lower birth weight as compared to Isolated Congenital Heart Diseases (ICHDs) [8]. Different studies have reported varying frequencies of ECAs in association with CHD. Karande S et al., found that craniofacial and skeletal anomalies were most commonly associated with heart defects [9]. While Tennstedt C et al., concluded that Central Nervous System (CNS) anomalies were most frequently seen with CHDs [10]. The various ECAs seen in association with CHDs are hydrocephalus, microcephaly, holoprosencephaly, agenesis of the corpus callosum, spina bifida, oesophageal atresia, duodenal atresia, anal atresia, diaphragmatic hernia, omphalocele, cleft lip/palate, polydactyly, syndactyly, club foot, renal dysplasias and pulmonary hypoplasia [7].
Aim of study was to evaluate the role of fetal echocardiography in prenatal screening of CHDs, to detect and correlate other associated extracardiac anomalies.
Materials and Methods
This was a prospective correlative type of study done over a period of 12 months from February 2015 to February 2016 on mid-trimester females who attended antenatal clinic in Government Medical College and Rajindra Hospital, Patiala, Punjab, India.
Inclusion criteria: Pregnancies with 13-28 weeks of gestation with viable foetuses were included.
Exclusion criteria: First and third trimester pregnancies, pregnancies with intrauterine fetal demise and threatened, incomplete abortions were excluded.
The Ethical Clearance from the Institute was obtained before starting the study and written informed consents were taken before enrolling the patients.
Fetal echocardiography was performed trans-abdominally using ‘Philips HD 11 XE’ machine with 2-5 Mhz broadband convex probe. The fetal echo views that were obtained were 4 chamber view, Left Ventricular Outflow Tract (LVOT), Right Ventricular Outflow Tract (RVOT), 3 Vessel view, 3 vessel trachea view, aortic arch view, bicaval view and ductal arch view. Standard fetal echocardiography was performed according to International Society of Ultrasound in Obstetrics and Gynaecology (ISUOG) guidelines.
Postnatal follow up was done in cases with abnormal findings on fetal echocardiography. Postnatal echocardiography was performed with 3-8 Mhz broadband phased array transducer. Autopsy confirmation was obtained in cases with Intra-Uterine Death (IUD).
Statistical Analysis
Data was expressed as Mean±SD. The statistical analysis was done by SPSS (16) using t-test and chi square test. A p-value <0.05 was considered statistically significant.
Results
A total of 1,200 pregnant women were included in study out of which 672 were low risk and 528 were high risk. Total 22 cases of CHDs were detected based on abnormal fetal echocardiography. On postnatal echo, four cases were found normal. These cases had minor anomalies like tiny membranous VSDs, small pericardial effusion and intracardiac echogenic focus detected on prenatal scan which were not found postnatally. From remaining 18 cases, in 15 cases (68.17%), there was complete agreement between the fetal echo findings and postnatal echo findings. In one case with antenatally detected muscular VSD, small peri-membranous VSD was missed which was detected on postnatal echo. [Table/Fig-1] shows the correlation between antenatal and postnatal findings in cases with CHDs.
Pie-chart showing correlation between prenatal and postnatal or autopsy findings in cases with congenital heart disease.
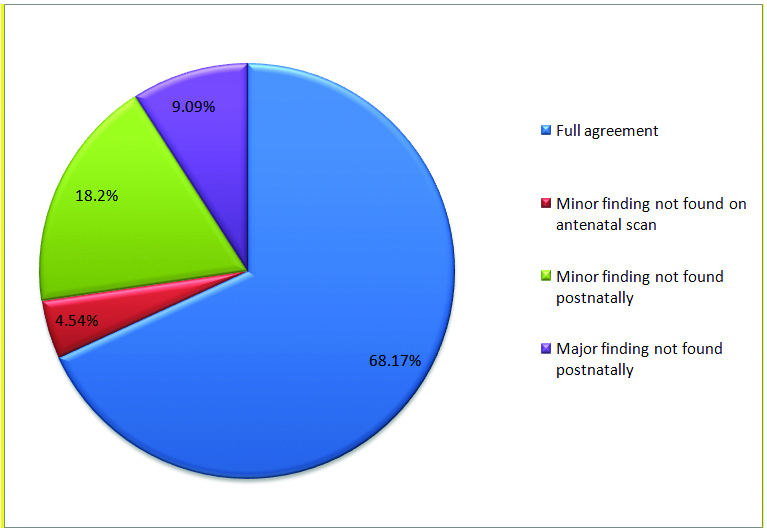
Eleven CHDs were detected in the low risk group and seven were found in the high risk group. [Table/Fig-2] shows the overall spectrum of CHDs detected. The incidence of prenatally diagnosed CHD in our study was 13.25 and 16.3 per 1,000 high and low risk pregnancies respectively which was not significant statistically. The overall incidence of CHDs was 15 per 1000 pregnancies. The mean maternal age of the study population was 27.61±4.68 years. The mean gestational age in present study was 20.37±4.25 weeks and the mean period of gestational age at which CHD’s were detected in fetuses was 22.72±3.30 weeks.
Distribution of congenital heart diseases in the present study.
| Diagnosis | No. of Patients | Percentage |
|---|
| Isolated VSD (IVSD) | 8 | 44.44 |
| VSD with Atrial Septal Defect (ASD) | 1 | 5.55 |
| Atrio-Ventricular Septal Defect (AVSD) | 3 | 16.66 |
| Ectopia Cordis | 1 | 5.55 |
| Transposition of Great Arteries (TGA) | 1 | 5.55 |
| TGA with Double Outlet Right Ventricle (DORV) | 1 | 5.55 |
| Hypoplastic Right Heart Syndrome (HRHS) | 1 | 5.55 |
| Truncus Arteriosus | 1 | 5.55 |
| Dextrocardia | 1 | 5.55 |
| Total | 18 | 100 |
The mean period of gestation at diagnosis of CHD cases with ECA was 22.8±3.27 weeks and the mean Period of Gestation (POG) at diagnosis of CHD cases without ECAs was 22.69 ±3.44 weeks. No significant difference was seen in the mean POG at diagnosis in these two groups.
IVSD was the most common CHD detected (44.44%). The mean POG at which IVSD’s were detected was 21.00±3.77 weeks. The second most common CHD detected was AVSD with mean POG at detection 25.66±1.15 weeks.
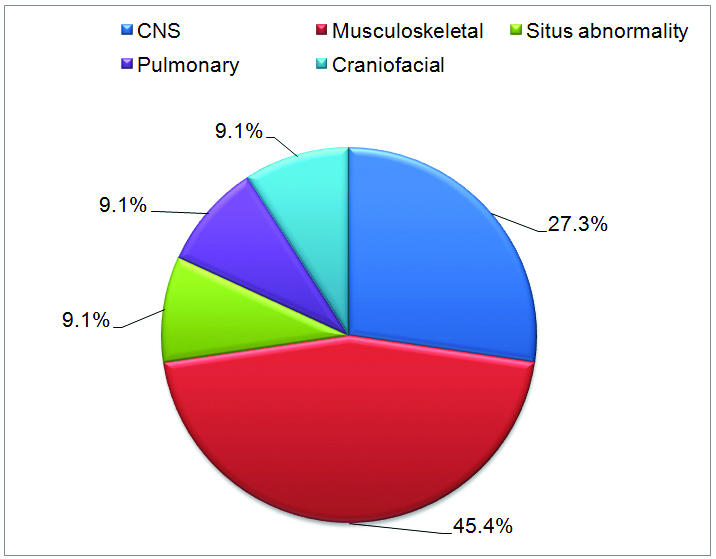
From 18 cases, five cases had associated ECAs. [Table/Fig-3] shows the frequency of associated ECAs in cases with CHD. Total 11 ECAs were found in these five cases indicating multisystem abnormalities.
Pie chart showing frequency of associated extracardiac anomalies (five cases).
[Table/Fig-4] shows the mean POG at diagnosis and outcome of abnormal cases. The cases with associated ECA had increased morbidity and born preterm.
The mean POG at diagnosis and outcome of pregnancies with CHD (t-test and chi-square test applied).
| CHD | Mean POG at diagnosis (weeks)Mean ± SD | POG at Birth (weeks)Mean ±S.D | Mean birth weight (kg) | Survival rate (post neonatal period) % |
|---|
| With ECA (n=5) | 22.8±3.27 | 36.00±1.15 | 2.32±0.150 | 40 |
| Without ECA (n=13) | 22.69±3.44 | 38.33±1.41 | 2.61±0.176 | 69.23 |
| p-value | 0.9517(NS) | 0.034(S) | 0.034(S) | 0.255 (NS) |
Out of 18 cases, 13 cases were delivered live from which four were associated with ECAs and nine were isolated CHDs. Two out of four live born cases with associated ECAs died in the neonatal period, while no neonatal death was seen in cases without associated ECA. IUD was seen in one case with ECAs. Out of 13 cases of CHD without ECA, IUD was seen in 4 (30.77%) cases. There were significantly more LSCS in cases with associated ECA. [Table/Fig-5] shows the mode of delivery in live born CHD cases. The overall survival rate seen in present study was 61.12%.
Shows the mode of delivery in live born cases with CHDS (LSCS-Lower Segment Cesarean Section, PTVD- Preterm Vaginal Delivery, FTVD-Full Time Vaginal Delivery, Chi square test applied).
| Mode | No. of Live Births (n=13) | p-value |
|---|
| With ECA(n=4) | Without ECA(n=9) |
|---|
| LSCS | 3 (75%) | 0 (0%) | 0.012 (S) |
| PTVD | 0 | 2 (22.22%) |
| FTVD | 1 (25%) | 7 (77.77%) |
Discussion
Due to the nature of the cardiac anatomy and the wide spectrum of defects, fetal heart is a difficult organ to examine. Also, the routine prenatal ultrasounds are not aimed at screening of fetal heart for CHDs. As a result, majority of CHDs go undetected in the fetal period. In present study conducted on 1200 mid trimester pregnant females, we correctly diagnosed 18 cases of fetal CHDs. The overall incidence of CHD in our study was 15 per 1,000. The incidence among high and low risk pregnancies was 13.25 and 16.3 per 1,000 respectively which is not significant statistically. In a study conducted by Nayak K et al., they reported a slightly higher incidence of CHDs detected by fetal echocardiography (20.3 per 1,000 pregnancies). The rates of occurrence of CHDs between high risk and low risk pregnancies were 16.9 and 22.3 per thousand respectively which was not statistically significant [11].
In our study, IVSD accounted for majority of CHDs (44.44%). This is in good correlation with study by Ozkutlu S et al., [12]. However, Nayak K et al., reported a higher frequency of endocardial cushion defects (19.2%) in their study [11].
The most frequent ECAs seen in association with CHDs in our study were of musculoskeletal system (45.4%). Similar result was seen in study by Calzolari E et al., [13].
Levi S et al., reported that ACHDs had lower gestational age at birth (36.0±3.5 weeks) as compared to ICHDs (37.2±3.3 weeks) [8]. Also, the mean birth weight was lower in CHDs with ECAs (2344±730 grams) as compared to isolated CHDs (2928±729 grams). Similarly, in our study, cases having CHD’s associated with ECA had lower POG at birth (36.00±1.15 weeks) and lower birth weight (2.32±0.150 kg) in comparison to CHD’s cases without ECA. Also, we found a statistically higher rate of LSCS (75%) in CHDs with associated ECA as compared to isolated CHDs. However, no statistically significant association was found between the rates of LSCS among the two groups by Levi S et al., [8].
The mortality rate in present study including IUDs and neonatal deaths was higher in cases with associated ECAs (60%) as compared to cases without ECAs (30.77%). Hsiao SM et al., also reported a higher mortality rate in CHD cases with associated ECAs [14].
We found complete agreement of 68.17% between prenatal echo findings and postnatal or autopsy findings which is comparable to studies by Noronha-Neto C et al., [15].
Limitation
The number of abnormal cases was very small due to the small sample size of the study. Also, postnatal follow up of each case could not be done, so sensitivity and specificity of fetal echocardiography could not be obtained. Our study should be interpreted within the confines of our limitation.
Conclusion
We suggest that fetal echocardiography should be included in the second trimester scan routinely in low as well as high risk pregnancies. The CHD with ECAs are associated with increased adverse outcome as compared to CHD without ECA.
[1]. Hoffman J, Kaplan S, The incidence of congenital heart diseaseJ Am Coll Cardiol 2002 39(12):1890-900. [Google Scholar]
[2]. Hoffman J, Congenital heart disease: incidence and inheritancePediatr Clin North Am 1990 37(1):25-43. [Google Scholar]
[3]. Garne E, Stoll C, Clement M, Euroscan Group, Evaluation of prenatal diagnosis of congenital heart diseases by ultrasound: experience from 20 European registriesUltrasound Obstet Gynecol 2001 17(5):386-91. [Google Scholar]
[4]. CarvalhoJ S, Mavrides E, Shinebourne EA, Campbell S, Thilaganathan B, Improving the effectiveness of routine prenatal screening for major congenital heart defectsHeart 2002 88(4):387-91. [Google Scholar]
[5]. Isaksen CV, Eik-Nes SH, Blaas HG, Tegnander E, Torp SH, Comparison of prenatal ultrasound and postmortem findings in fetuses and infants with congenital heart defectsUltrasound Obstet Gynecol 1999 13(2):117-26. [Google Scholar]
[6]. D’Vore G, The aortic and pulmonary outflow tract screening examinations in human fetusJ Ultrasound Med 1992 11(7):345-48. [Google Scholar]
[7]. Rosa RCM, Rosa RFM, Zen PRG, Paskulin GA, Congenital heart defects and extracardiac malformationsRev Paul Pediatr 2013 31(2):243-51. [Google Scholar]
[8]. Levi S, Zhang WH, Alexander S, Viart P, Grandjean H, Eurofetus study group. Short-term outcome of isolated and associated congenital heart defects in relation to antenatal ultrasound screeningUltrasound Obstet Gynecol 2003 21(6):532-38. [Google Scholar]
[9]. Karande S, Patil V, Kher A, Muranjan M, Extracardiac birth defects in children with congenital heart defectsIndian Pediatrics 2014 15:389-91. [Google Scholar]
[10]. Tennstedt C, Chaoui R, Korner H, Dietel M, Spectrum of congenital heart defects and extracardiac malformations associated with chromosomal abnormalities: Results of a seven year necropsy studyHeart 1999 82:34-39. [Google Scholar]
[11]. Nayak K, Chandra GSN, Shetty R, Narayan PK, Evaluation of fetal echocardiography as a routine antenatal screening tool for detection of congenital heart diseaseCardiovascular Diagnosis and Therapy 2016 6(1):44-49. [Google Scholar]
[12]. Ozkutlu S, Akca T, Kafali G, Beksac S, The results of fetal echocardiography in a tertiary center and comparison of low- and high-risk pregnancies for fetal congenital heart defectsAnatol J Cardiol 2010 10(3):262-69. [Google Scholar]
[13]. Calzolari E, Garani G, Cocchi G, Magnani C, Rivieri F, Neville A, Congenital heart defects: 15 years of experience of the Emilia-Romagna Registry (Italy)Eur J Epidemiol 2003 18:773-80. [Google Scholar]
[14]. Hsiao SM, Wu MH, Jou HJ, Lee CN, Shyu MK, Shih JC, Outcome for fetuses with prenatally detected congenital heart disease and cardiac arrhythmias in TaiwanJ Formos Med Assoc 2007 106(6):423-31. [Google Scholar]
[15]. Noronha-Neto C, Souza AS, MoraesFilho OB, Noronha AM, Validation of ultrasound diagnosis of fetal anomalies at a reference center in PernambucoRev Assoc Med Bras 2009 55(5):541-46. [Google Scholar]